
Glycosides of fatty acids
with a N-acyl link
In these glycosides, the fatty acids may have only N-acyl links or one N-acyl link and one acyl link.
Lipo-chitooligosaccharides or Nod factors
The close association between symbiotic bacteria (or Rhizobia) and leguminous plants leads to the formation of nitrogen-fixing nodules, conferring on host plants the ability to fix nitrogen. This symbiosis is characterized by a high level of host specificity, mediated by specific recognition of rhizobial molecules called Nod factors (Cullimore JV et al., Trends plant sci 2001, 6, 24).
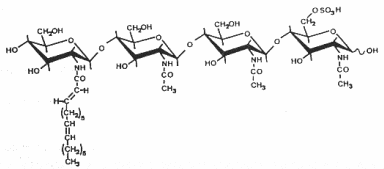
The structure and the specific functions of these "nodule-inducing principle" (Truchet G et al., Differentiation 1980, 16, 163) were discovered in Rhizobium meliloti (Lerouge P et al., Nature 1990, 344, 781). These are lipo-chito-oligosaccharides, based on a backbone of generally four or five N-acetyl glucosamine residues (chitine oligomer) which are N-acylated at the non-reducing end and carry various substitutions which are important determinants of rhizobial host specificity, the terminal non-reducing sugar being N-acylated with a fatty acid of 16 to 18 carbon residues. Different species of Nod factors can be substituted with acetate, sulfate, or carbamoyl groups, or can have different sugars, such as arabinose, mannose, or fucose. The degree of saturation of the acyl chain can also vary from 0 up to 4 double bonds (Perret X et al., Microbiol Mol Biol Rev 2000, 64, 180).
As the legume-rhizobia symbiosis is established, the plant recognizes Nod factors and initiates transcriptional and developmental changes within the root to allow bacterial invasion and the construction of a novel organ, the nodule. Nod factors thus act as symbiotic signalling molecules for conferring host specificity, for infection, and for nodule development (Hirsch AM et al., Plant Physiol 2001, 127, 1484), and are likely to be perceived by high affinity receptors (Cohn J et al., Trends Plant Sci 1998, 3,105).
A review of the nod-factor signaling has been read for further details (Limpens E et al., Curr Opin Plant Biol 2003, 6, 343).
Lipid X
Lipid X has been identified as the monosaccharide precursor in the biosynthesis of lipid A, and thus LPS, and of Gram-negative endotoxin (Takayama K et al., J Biol Chem 1983, 258, 7379). It is available by isolation from phosphatidylglycerol-deficient mutants of Escherichia coli (Ray B L et al., J Biol Chem 1984, 259, 4852) and also by synthetic routes.
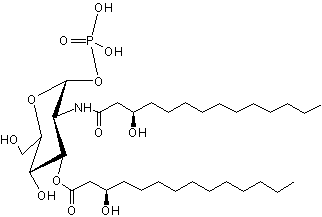
Lipid X
Lipid X has been reported to directly induce cytokine release in macrophages but also to inhibit endotoxin induced tumor necrosis factor induction. Very pure lipid X was shown to induce TNF release and cytotoxicity against tumor cells in murine macrophages (Aschauer H et al., J Biol Chem 1990, 265, 9159). Furthermore, Lipid X was shown to compete with lipopolysaccharides in order to inhibit neutrophiles stimulation (Danner RL et al., J Clin Invest 1987, 80, 605). Thus, lipid X is able to enhance survival in animal models of severe infection with gram-negative organisms ( Golenbock DT et al., Infect Immun 1988, 56, 779).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire