
This class, known also as ethynoic acids, includes fatty acids which contain a triple bond (or more as in polyacetylenes) and eventually one or two double bonds. While many acetylenic fatty acids have been prepared synthetically, only some species are found in natural oils.
Acetylenic fatty acids (tariric acid) were discovered in the seed fat of Picramnia Sow (Simarubaceae) in the 19th century by the French chemist Arnaud A (Bull soc chim 1892, 7, 233). They appear to be common in tropical plants mainly belonging to the order of Santalales including the 2 families Santalaceae and Olacaceae. These fatty acids form up to 90% or 50% of the seed fat of the Santalaceae or Olacaceae, respectively.
We give below some of the best known acetylenic fatty acids found in plants:
Stearolic acid (9-octadecynoic acid) is present in seeds of Santalaceae.
Tariric acid (6-octadecynoic acid) was first reported in the seed fat of the Simarubiaceae Picramnia tariri (Arnaud A, C R Acad Sci 1892, 114, 79) an later in other Picramnia species. Among them, the seed oil from Picramnia sow, a plant indigenous to Guatemala, has been reported to contain nearly 95% of tariric acid (Steger A et al., Rec Trav Chim 1933, 52, 593).
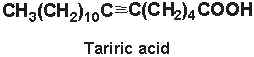
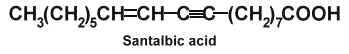
Santalbic acid (or Ximenynic acid) has been described in the seed fat of Santalum album at high concentration (50%) (Madhuranah MK et al., J Indian Chem Soc 1938, 15, 389) and its structure was later identified (Gunstone FD et al., Chem Ind 1954, 1112).
The two acetylenic fatty acids, santalbic acid and stearolic acid, were isolated from the seeds of Exocarpus and Santalum (Morris LJ et al., Chem Ind 1966, 12, 460). This distinctive biochemical feature was discovered in santalwood oil from a small tree or shrub (Santalum spicatum) native to the Western Australia (Hatt HA et al., J Sci Food Agric 1956, 7, 130). Its large fruit contain a hard-shelled seed rich in a drying fixed oil (50-60%). About 30-35% of total fatty acids are represented by santalbic acid and 1% by stearolic acid. When administered to animals these fatty acids were described to inhibit several lipoenzymes (lipoxygenase, prostaglandin synthetase) and modify tissue fatty acid composition (Liu Y et al., Lipids 1997, 32, 965).
Santalbic acid rich oils are used as a skin cosmetic to improve skin hydration and elasticity and UV screen. It has been proved that santalbic acid has also anti-aging properties. It has been demonstrated that it has anti-inflammatory properties acting as a potent inhibitor of the eicosanoids synthesis, via the inhibition of COX-1.
Two acetylenic acids (6-octadecynoic and 6-nonadecynoic acids) were described in the roots of a Peruvian plant (Pentagonia gigantifolia, Rubiaceae) and were shown to inhibit the growth of fluconazole-susceptible and -resistant Candida albicans strains (Li XC et al., J Nat Prod 2003, 66, 1132). Their antifungal potencies were reported to be comparable to those of amphotericin B and fluconazole and to have low cytotoxicity.
6,9-octadecenynoic acid (6-octadecen-9-ynoic acid) was isolated from nuts of Ongokea klaineana. This compound contains a triple-bond at carbon 9 and a double bond at carbon 6.
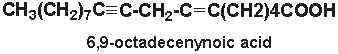
Pyrulic acid (t10-heptadecen-8-ynoic acid) was isolated first from seed oil from a Santalaceae Pyrularia pubera, and recently found in a Olacaceae from southern Brazil, Heisteria silvanii (Spitzer V et al., Lipids 1997, 32, 1189).
Crepenynic acid (9-octadecen-12-ynoic acid) was found in high concentration (60%) in oil extract from seeds of Crepis foetida (Compositae) (Mikolajczak KL et al., J Org Chem 1964, 29, 318). Toxic effects of plants containing this fatty acid have been reported in Australian sheep.
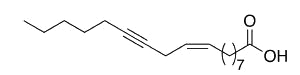
Crepenynic acid
The distribution of crepenynic acid and dehydrocrepenynic acid (9,14-octadecen-12-ynoic acid) (65% of total fatty acids) in triacylglycerols of the aril and cotyledon oils of Afzelia cuanzensis (Caesalpinaceae) was studied by nuclear magnetic resonance spectroscopy (Vlahov G, Phytochemistry, 1996, 42, 621).
The oil from the seed of Alvardoa amorphoides (Simarubiaceae) was reported to contain about 15% of 17-octadec-6-ynoic acid, the predominant fatty acid of this oil being tariric acid (Pearl MB et al., Lipids 1973, 8, 1284). Crepenynic acid was also observed at levels of 18% in the seeds of Atractylodes lancea and 13–15% in A. macrocephala, species largely used in traditional Chinese medicine (Sun JY et al., JAOCS 2017, 94, 655).
Similar acetylenic acids, but with 14 to 18 carbon atoms and one to three hydroxyl groups have been described in the fungus Coriolopsis gallica (basidiomycete) (Zhou ZY et al., J Nat Prod 2008, 71, 223).
Two acetylenic acids with one triple double bond and one double bond were described in a Santalaceae, Nanodes muscosa from Chili. One of them (compound 2) constitutes the first example of an a,w fatty diacid with a conjugated enzyme system from a natural source (El-Jaber N et al., J Nat Prod 2003, 66, 722).
Scleropyric acid was isolated from another Santalaceae, Scleropyrum wallichianum (Suksamrarn A et al., Chem Pharm Bull 2005, 53, 1327). That acetylenic acid exhibited antimycobacterial (MIC : 25 mg/ml) and antiplasmodial (MIC : 7.2 mg/ml) activities.
![]()
Scleropyric acid
An acetylenic fatty acid with ene-yne-ene structure was described in an Olacaceae, Heisteria silvanii (popular name “casca-de-tatu): heisteric acid (Spitzer V et al., Lipids 1997, 32, 1189). It has been tentatively characterized by their mass spectra as cis-7,t11-octadecen-9-ynoic acid and determined to present at a level of about 23% in seed oil
![]()
A conjugated t,t-diunsaturated acetylenic acid was found as a main component in the seed oil of an ornemental Asteraceae, Tanacetum (Chrysanthemum) corymbosum (Compositae). This fatty acid was shown to be t8, t10-octadecadien-12-ynoic acid (Tsevegsuren N et al., Lipids 1998, 33, 723).
Acetylenic acids were also described in sponges. Several compounds with a branched-chain, an acetylenic moiety in the middle of the molecule and a terminal double bond, and sometimes with a methoxy group were described in a marine sponge Stellettaspecies (Lee HS et al., J Nat Prod 2003, 66, 566; Zhao Q et al., J Nat Prod 2003, 66, 408).
Polyacetylene fatty acids are not commonly found in living organisms. Mycomycin, which is produced by a mold-like Actinomycete and is active against the Bacilli sp of human tuberculosis was discovered in 1947 by Johnson EA et al. (J Bacteriol 1947, 54, 281). This fatty acid has two triple bonds but has also an allenic structure : tridecatetra-3,5,7,8-en-10,12-diynoic acid.
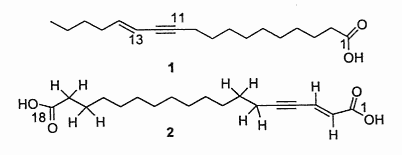
A diynoic acid (13,14-dihydrooropheic acid) has been isolated from an Indonesian plant (Mitrephora celebica, Annonaceae) and was shown to have a potent antimicrobial activity (Zgoda J et al., J Nat Prod 2001, 64, 1348).
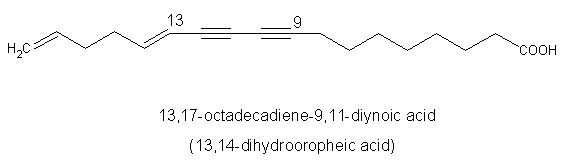
Another diynoic acid (octadecen-13-en-9,11-diynoic acid) has been described in Santalum acuminatum and also in Nanodea muscosa (Santalaceae) where its exact structure was elucidated (El-Jaber N et al., J Nat Prod 2003, 66, 722). Several others have been described in Olacaceae : octadeca-13-en-9,11-diynoic acid (exocarpic acid), octadeca-13,17-dien-9,11-diynoic acid, octadeca-17-en-9,11-diynoic acid (isanic acid), and octadeca-9,11-diynoic acid (Badami RC et al., Prog Lipid Res 1981, 19, 119).
A review of several polyacetylenic acids in plants and the current state of knowledge of the biochemistry and molecular genetics of polyacetylenic metabolic pathways may be consulted (Minto RE et al., Prog Lipid Res 2008, 47, 233).
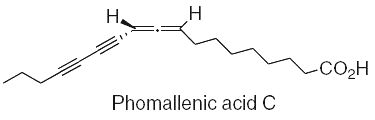
Three fatty acids with an allenyldiyne structure, phomallenic acids A-C, have been isolated from the fermentation broth of Phoma sp. Phomallenic acid C had the most potent activity as an inhibitor of bacterial FAS II pathway (Ondeyka JG et al., J Nat prod 2006, 69, 377).
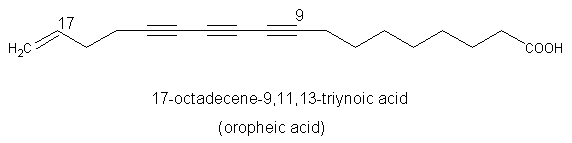
A triynoic acid (oropheic acid) was isolated from leaves of Orophea enneandra (Annonaceae)(Cavin A et al., J Nat Prod 1998, 61, 1497) and of Mitrephora celebica (Zgoda J et al., J Nat Prod 2001, 64, 1348). It displayed a significant activity against the fungus Cladosporium.
A 9-carbon triynoic acid containing a terminal acetylene has been isolated first from the Basidiomycetes Psilocybe sarcocephala (Jones ERH, Proc Chem Soc 1960, 199) but was later found in other fungi of the same group. An amide of that fatty acid with p-aminobenzoic acid has been shown to be present in the fungus Baeospora myosura and to have a selective antibacterial activity (Parish CA et al., J Nat Prod 2004, 67, 1900). Triacetylenic acids, named corticatic acids, have been isolated from the marine sponge Petrosia corticata. One of them is shown below. All are geranylgeranyltransferase type I (from Candida albicans) inhibitors (Nishimura S et al., J Nat Prod 2002, 65, 1353).
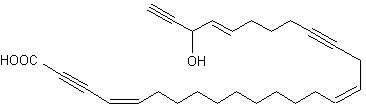
Corticatic acid A
Another triynoic acid with 20 carbon atoms, from synthetic origin, is frequently used to experimentally inhibit essential fatty acid metabolism and especially cyclooxygenase and lipoxygenase pathways: 5,8,11,14-eicosatetraynoic acid (ETYA).
A bromated triynoic acid lipid was isolated from the Chinese marine sponge Xestospongia testudinaria, which was shown to inhibit protein tyrosine phosphatase 1B, considered as a significant target for the “treatment of type II diabetes and obesity” (He WF et al., J Asian Nat Prod Res 2015, 17, 861).
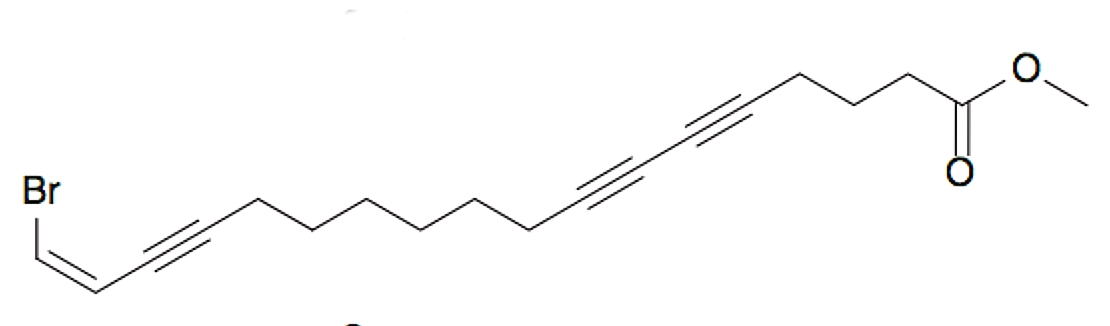
Xestospongia triynoic acid
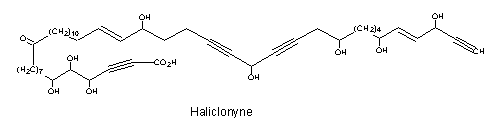
A tetraynoic acid (Haliclonyne) was isolated from the marine sponge Haliclona collected at Eilat (Israel) (Chill L et al., J Nat Prod 2000, 63, 523). This highly polyacetylene compound is a C47 oxo-octahydroxy-dientetrayne carboxylic acid.
New molecules, named osirisynes, have been also isolated from the sponge Haliclona sp collected in Mayotte (Campos PE et al., Mar. Drugs 2020, 18, 350). They were tested as inhibitor of proteasome kinase, CDK7 and Fyn kinase.
Another tetraynoic acid, named protegenin, is produced by a strain of Pseudomonas protegens as a weapon in the continual competition between microbes (Murata K et al., ACS Chem Biol 2022, 17, 3313).
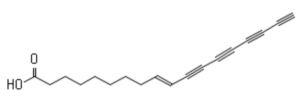
Protegenin A
Similar families of polyyne lipids have been discovered in other bacteria, for example the Caryoynencins from Trinickia caryophylli.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire