
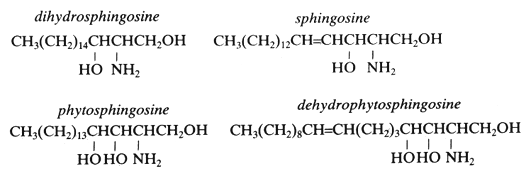
This family of simple lipids is mainly found as homologues of the most spread base sphingosine, a C18-aminodiol. Sphingosine was discovered around 1880 by Thudichum J but its structure was elucidated in 1947 (Carter HE et al., J Biol CHem 1947, 170, 285). Phytosphingosine is the counterpart of sphingosine in the plant world. This compound was isolated by Reindel in 1930 from yeast (Reindel F, Ann Chem 1930, 480, 76), and by 1940 its structure has been partially elucidated. Its distribution is not exclusively in plants since it was also detected in animal tissues in 1964 (Karlsson KA, Acta Chem Scand 1964, 18, 2397). A review gives an overview of the naturally occurring and synthetic sphingoid base-like compounds (Pruett ST et al., J Lipid Res 2008, 49, 1621).
All these amino alcohols occur largely in complex form (amides of fatty acids) as sphingolipids (ceramides, sphingomyelin, cerebrosides and complex glycolipids). More than 60 long-chain bases were described in bacteria, plants and animals with 12 to 20 carbon atoms , 2 to 3 hydroxy groups and zero to 2 double bonds, some may be phosphorylated or sulfated.
Complex poly amino alcohols with a long alkyl chain unrelated to sphingosine-type compounds are found in marine sponges.
The simple amino alcohols are frequently represented by a simplified nomenclature similar to that used for fatty acids but with additional d or t to designate di- and trihydroxy bases, respectively. The most common amino alcohols are given in the table below:
|
Name |
Formula |
PM |
Chemical name |
| sphingosine d18:1 |
C18H37NO2 |
299 |
4-sphingenine (2D-aminooctadec-t-4-ene-1,3-diol) |
| dihydrosphingosine d18:0 |
C18H39NO2 |
301 |
sphinganine (2D-aminooctadecane-1,3-diol) |
| C20-dihydrosphingosine d20:0 |
C20H43NO2 |
329 |
eicosasphinganine (2D-aminoeicosane-1,3-diol) |
| phytosphingosine t18:0 |
C18H39NO3 |
317 |
4-hydroxysphinganine (2D-aminooctadecane-1,3,4-triol) |
| C20-phytosphingosine t20:0 |
C20H43NO3 |
345 |
4-hydroxyeicosasphinganine (2D-aminoeicosane-1,3,4-triol) |
| dehydrophytosphingosine t18:1 |
C18H37NO3 |
315 |
4-hydroxy-8-sphingenine (2D-aminooctadec-t-8-ene-1,3,4-triol) |
| sphingadienine d18:2 |
C18H35NO2 |
297 |
4,8-sphingadienine (2D-aminooctadeca-4,8-diene-1,3-diol) |
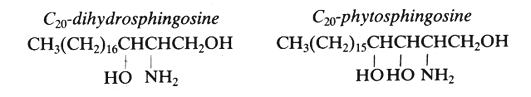
The initial step of the de novo biosynthesis of sphingosine is the condensation of serine with palmitoyl-CoA to form 3-ketodihydrosphingosine. This product is rapidly reduced by a NADPH-dependent reductase to dihydrosphingosine. Recent evidence suggests that this amine is first acylated by a fatty acyl-CoA to give a dihydroceramide which will be further converted to ceramide, the precursor of all sphingolipides, by the introduction of a trans-4,5-double bond. The demonstration of a differential regulation of that last step by palmitate versus oleate provides insight into the mechanisms of oleate-mediated protection against diabetes and metabolic disease (Hu W et al., J Biol Chem 2011, 286, 16596). Free sphingosine is only present in cell at very low concentrations since it is now considered as a lipid mediator. It was discovered that sphingosine (and other long-chain bases) inhibit protein kinase C in vitro and the common cellular responses to this enzyme (cell proliferation, platelet aggregation, neutrophile respiratory burst, differentiation of leukemic cells…) (review in : Hannun YA et al., Nature rev Mol Cell Biol 2008, 9, 139).
A review on this important properties appeared in Biochimica (Moscow). A review on the sphingoid bases biosynthesis has been released (Merrill AH, J Biol Chem 2002, 277, 25843).
Sphingadienine induces colon cancer cell death in vitro and prevent intestinal tumorigenesis in vivo (Fyrst et al., Cancer Res 2009, 69, 9457). Sphingadienines exert their influence by blocking Akt translocation from the cytosol to the membrane, thereby inhibiting protein translation and promoting apoptosis and autophagy. Thus, that compound may represent a new class of therapeutic and/or chemopreventive agents.
An analog of sphinganine (or dihydrosphingosine) but with 17 carbon atoms, present in bacteria-contaminated mussels, has been shown to be associated with a strong toxicity (diarrheic and paralysis symptoms) (Marrouchi R et al., Mar Drugs 2013, 11, 4724).
1-Deoxysphinganine (or Spisulosine) is a bioactive sphingoid, sphinganine extracted from the arctic surf clam Spisula polynyma in which the terminal hydroxy group has been replaced by a hydrogen. It has a role as an antineoplastic agent in preclinical studies. It inhibits the growth of the prostate PC-3 and LNCaP cells through intracellular ceramide accumulation and PKCζ activation. It is both a sphingoid and an amino alcohol. It is functionally related to a sphinganine.
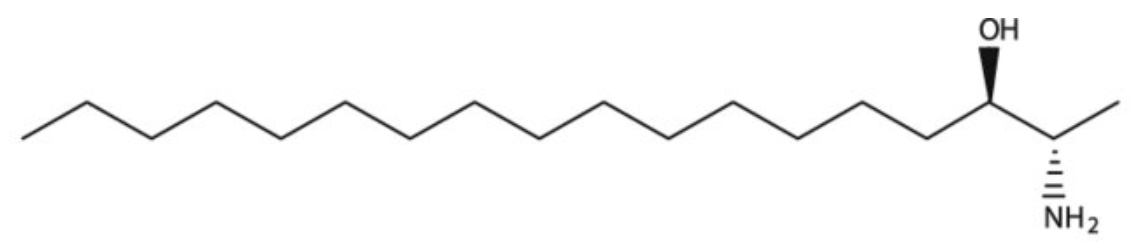 Spisulosine
Spisulosine
The first and rate-limiting step of the long-chain base synthesis is catalyzed by the enzyme serine-palmitoyltransferase (SPT), which typically conjugates L-serine and palmitoyl-CoA. The product, 3-keto-sphinganine, is rapidly converted to sphinganine (d18:0). Other fatty acyl-CoAs will form sphingoid bases with variable carbon chain length in the range of C14 to C22. Moreover, SPT shows an alternative activity towards other amino acid substrates, in particular, for alanine and glycine, which results in the formation of an atypical category of 1-deoxy-sphingolipids. The conjugation with L-alanine forms 1-deoxysphinganine, while the use of glycine will form 1-deoxymethyl-sphinganine.
The accumulation of two neurotoxic amino alcohols, 1-deoxy-sphinganine and 1-deoxymethyl-sphinganine, were shown to be associated with an inherited sensory neuropathy (HSAN1) (Penno A et al., J Biol Chem 2010, 285, 11178). Both metabolites lack the C1 hydroxyl group of sphinganine. One of these compounds is shown below.
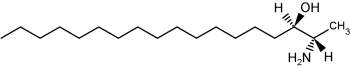
1-Deoxy-sphinganine
1-Deoxy-sphingolipids are evolutionary conserved and have been found in several fungal species and lower invertebrates such as molluscs and sponges. However, for a long time it was unknown that these metabolites could be found in mammals. 1-Deoxysphinganine was first isolated from the marine clam Spisula polynyma as an anti-cancer compound (Cuadros R et al., Cancer Lett 2000, 152, 23). Later, two independent studies identified the same molecule as an endogenously formed lipid metabolite in mammals. The first was during a study in the context of a rare neuropathy (Penno A et al., J Biol Chem 2010, 285, 11178). A review may be consulted on that topic (Lone MA et al., Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 512).
Dihydrosphingosine (dhSph), dihydrosphingosine-1-phosphate (dhS1P) and dihydroceramide (dhCer) are dihydrosphingolipids which do not contain a C4-trans-double bond in the sphingoid backbone. These compounds have received less attention and research is lacking especially in terms of their mechanisms of action. Despite that situation, it has been shown that the presence of dhCer may predict type 2 diabetes in obese individuals, dhS1P predicting cardiovascular diseases and dhSph predicting hepato-renal toxicity. A review gives a comprehensive summary of research in the last 10–15 years on the dihydrosphingolipids and their relevant roles in different diseases (Magaye RR et al., Cell Mol Life Sci 2019, 76, 1107).
Two antifungal compounds, designated sphingofungin B and C, isolated from a culture of Aspergillus fumigatus were shown to have broad spectrum antifungal activity, but little or no antibacterial activity (Van Middlesworth et al., J Antibiot 1992, 45, 861).
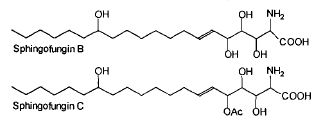
The structure of these compounds revealed a resemblance to the long chain bases found in sphingolipids. They may be described as amino fatty acids or carboxylated long chain base. This resemblance is in agreement with the demonstration of an inhibition of the first committed enzyme of sphingolipid biosynthesis, serine palmitoyltransferase (ZweerinkMM et al., J Biol Chem 1992, 267, 25032).
Another amino fatty acid, myriocin, was discovered as an antibiotic from the culture broth of a fungus, Myriococcum albomyces (Klüpfel D et al., J Antibio 1972, 25, 109). Later, it was also isolated from the culture broth of Isaria sinclairii, a mushroom used in Chinese folk medicine which has potent immunosuppressive properties (Fujita T et al., J Antibiot 1994, 47:208 ).
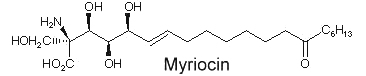
The chemical modification of myriocin (2-alkyl-2-aminopropane-1,3-diol) yielded a new compound, FTY720 (fingolimod), which was highly effective in experimental allotransplantations and against autoimmune diseases (multiple sclerosis, rheumatoid arthritis) (Chiba K, Pharmacol Ther 2005, 108, 308).
An anhydrophytosphingosine, with a furan group, named pachastrissamine, has been isolated as a cytotoxic principle of a marine sponge, Pachastrissa sp (Kuroda I et al., J Nat Prod 2002, 65, 1505). The tetrafuran group and the saturated chain likely derived from a phytosphingosine precursor.
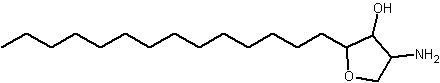
Pachastrissamine
Similar sphingoid bases have been isolated from another marine sponge, Penares sp (Ando H et al., J Nat Prod 2010, 73, 1947). They have unsaturated chain, methylated or not, of 24 or 26 carbon atoms. They exhibit moderate cytotoxicity against HeLa cells.
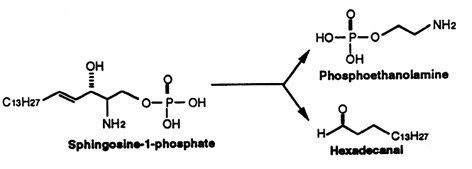
Sphingosine (and sphinganine) can be converted to sphingosine-1-phosphate (S1P)(and sphinganine-1-phosphate) by a specific kinase. In 1970, Stoffel (Hoppe-Seyler’s Z Physiol Chem 1970, 351, 635) reported the formation of S1P in erythrocytes, a kind of phosphosphingolipid which appeared later involved in cellular proliferation. Indeed, Olivera A et al. demonstrated that S1P plays the role of a second messenger in cell proliferation induced by PDGF and FCS mitogens (Olivera A et al., Nature 1993, 365, 557). Later, SIP was demonstrated to be a ligand for the G protein-coupled receptor EDG-1 (Lee M J et al., Science 1998, 279, 1552). Several historical details may be discovered in an invited paper published in JBC (Spiegel S, J Biol Chem 2020, 295, 3371).
S1P is classified here as simple lipid since formed of one component and a phosphate group. S1P is normally present in low abundance under normal physiological conditins (about 0.1 mol% of total cell lipids). It was recently shown that this compound is rapidly produced in response to mitogenic concentrations of sphingosine. It can, at very low concentrations, induce increased DNA synthesis and cell division in some cells but is also a potent growth inhibitor for some human breast cancer cell lines. Many experiments have shown that S1P is involved in the regulation of the traveling of lymphocytes T and B (lymphocyte egress) from thymus to peripheral lymphoid organs, an important step of the adaptive immunity (Matloubian M et al., Nature 2004, 427, 355). Curiously, important amounts of S1P were detected in human platelets. Furthermore, mounting evidence indicates that multiple antiatherogenic or anti-inflammatory actions of lipoproteins HDL independent of cholesterol metabolism are mediated by the lipoprotein-associated S1P through its receptors (Okajima F et al., Endocr J 2009, 56, 317). Further investigations indicate that HDL-associated S1P is responsible for the beneficial effects of these lipoproteins on vasorelaxation, cell survival, cell adhesiveness, angiogenesis and synthesis of two powerful endogenous anti-atherogenic and anti-thrombotic molecules such as nitric oxide (NO) and prostacyclin (PGI2) (Rodriguez C et al., Thromb Haemost 2009, 101, 665). New data proposes S1P as an essential second messenger, whose synthesis is enhanced by photoreceptor trophic factors, such as GDNF (glial derived neurotrophic factor) and docosahexaenoic acid, in order to control key processes in the development of photoreceptors (Rotstein NP et al., J Lipid Res 2010, 51, 1247).
This compound can be cleaved to ethanolamine phosphate and t-2-hexadecanal by a pyridoxal phosphate lyase. This ethanolamine phosphate may be used for the synthesis of phospholipids such as phosphatidyl ethanolamine and phosphatidyl choline, the fatty aldehyde being used for the synthesis of plasmalogens or fatty acids.
Sphinganine-1-phosphate (or dihydrosphingosine-1-phosphate) has also been found to bind to sphingosin-1-phosphate receptors, but its binding ability is less potent than that of its homologue (Tamama K et al., Biochem J 2001, 353, 139). That derivative has been shown to be involved in the stimulation of metalloproteinase 1 in association with tumor growth and metastasis (Bu SM et al., FASEB J 2006, 20, 184).
A study has defined a novel role for the sphingosine phosphate in phosphate-induced vacular matrix mineralization and has shown that blocking specific metabolic pathways reduced vascular mineralization (Morris TG et al., J Lipid Res 2018, 59, 69). These results may inform new therapeutic approaches to inhibit or delay vascular calcification.
Comprehensive reviews of S1P and other sphingolipid mediators have been released (Fyrst H et al., Nature Chem Biol 2010, 6, 489; Hannun YA et al., Nature rev Mol Cell Biol 2008, 9, 139) and its important regulatory properties can be found in Biochimica (Moscow) and in papers by Hla T (Sem Cell Develop Biol 2004, 15, 513) and by Takuwa Y (Biochim Biophys Acta 2008, 1781, 483) and Ksiazek M (J Lipid Res 2015, 56, 1271).
In plants, a role for S1P has been shown in drought stress and stomacal guard cell closure, linking the phosphorylated lipid-derived signal to this calcium-mediated process (Ng C et al.,Nature 2001, 410, 596). Moreover, intracellular S1P plays an important role as an intermediate of the sphingolipid-to-glycerophospholipid metabolic pathway (review in Kihara A, Biochim Biophys Acta 2014, 1841, 766).
Sphingosine sulfates have been isolated from a marine sponge (Spirastrella abata) and were shown to exhibit significant cytotoxicity against tumor cell lines (Alam N et al., J Nat Prod 2002, 65, 944). Two homologue structures were elucidated, the carbon chain having either 17 or 18 carbon atoms, the sulfate group at the C-4 position and the double bond being at the C-6 position (instead of the C-4 position in the sphingosine molecule).
An unusual sulfonated derivative of a 17-carbon amino alcohol was shown to be a major component of the cell envelope of gliding bacteria of the genus Cytophaga and of closely related genera (Godchaux W et al., J Bacteriol 1980, 144, 592). This lipid, named capnine, a structural analogue of sphinganine, was purified and was shown to be 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid. N-acylated derivatives of capnine were also described.
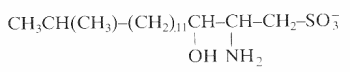
Similar sulfonolipids have been described in diatoms (Anderson R et al., Biochim Biophys Acta 1978, 528, 89).
A carboxylated capnine (halocapnine) is part of a ceramide analogues found in halophilic bacteria, the acylhalocapnines (Baronio M et al., J Lipid Res 2010, 51, 1878).
Branch-chain sphingoid bases have been described in some marine invertebrates. Thus, a base with a branched C19 alkyl chain and three double bonds, 2-amino-9-methyl-4,8,10-octadecatriene-1,2-diol, was shown to be present in glucosylceramide from starfish (Irie A et al., J Biochem 1990, 107, 578) and in sphingomyelin from squid nerve (Ohashi Y et al., J Lipid Res 2000, 41, 1118). A branched base with two double bonds has been found in cerebrosides from a sea anemone (Karlsson KA et al., Biochim Biophys Acta 1979, 574, 79) and from mycelia of a fungus (Kawai G et al., J Lipid Res 1985, 26, 338). The presence of these branched and unsaturated sphingoid bases in primitive organisms may indicate an essential role in their cell membranes.
While the glycosphingolipids in plants and animals share a sphingoid base as a common feature, a dimeric amino alcohol base was shown to be present in certain calcareous sponges. These compounds have a symmetrical, or almost symmetrical, long hydrocarbon chain (C28-C30) with at both ends a vicinal amino alcohol.
Two of these typical dimeric molecules, leucettamol A and B, have been described in a free state in a sponge from Micronesia Leucetta microraphis (Kong FH et al., J Org Chem 1993, 58, 970). One of these 2-amino-3-hydroxy hydrocarbons is shown below
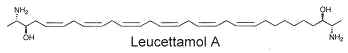
These molecules were shown to have only mild antibacterial activity. The absolute configuration of leucettamol A has been determined by precise physical methods (Dalisay DS et al., J Nat prod 2009, 72, 353).
Parent compounds containing a 1,3-diamino-2-propanol moiety, coriacenins, have been described in a Mediterranean sponge Clathrina coriacea (Casapullo A et al., J Org Chem 1996, 61, 7415).
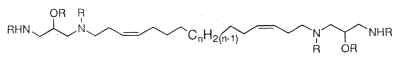
n = 9, 10 or 11; R = H or Ac
Another one, rhapsamine, was extracted in an Antarctic sponge Leucetta leptorhapsis (Jayatilake GS et al., Tetrahedron Lett 1997, 38, 43, 7507). It has the structure of a linear C28 polyene terminally substituted by 1,3-diaminoglycerol groups and has cytotoxic activity.
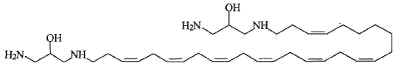
A related molecule has been described in an Australian calcareous sponge (class Calcarea) and named BRS1 (Willis RH et al., Toxicon 1997, 35, 1125).
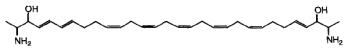
Others, crucigasterins, were described in a Mediterranean tunicate Pseudistoma crucigaster (Jares-Erijman EA et al., J Org Chem 1993, 58, 5732).
Oceanapiside is a glycosylated “two-headed” α,ω-bis-amino alcohol with chain termini that resemble the functional head groups of sphinganine (dihydrosphingosine) and fumonisins. It is fungicidal against fluconazole-resistant Candida glabrata at 10 μg/ml in targeting the sphingolipid pathway of the fingus (Dalisay DS et al., Mar Drugs 2021, 19, 126).
All these molecules were shown to have various cytotoxic activities. Furthermore, they can be glycosylated thus forming simple “sphingolipids” with potent cytotoxic properties.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire