
Epoxy acids are present in a number of seed oils (in triacylglycerols) and in plant cutin and suberin. The natural species are all C18 compounds, saturated on unsaturated. On prolonged storage, they can be formed as hydroxy derivatives. For example, 9,10-epoxystearic and 9,10-epoxyoctadec-12-enoic (coronaric acid) acids are found in sunflower seeds (Chrysanthemum). The seed oil of Bernardia pulchella (Euphorbiaceae) contains 91% of coronaric acid (Spitzer V et al., JAOCS, 1996, 73, 1733). These fatty acids are commonly used to make paints and coatings.
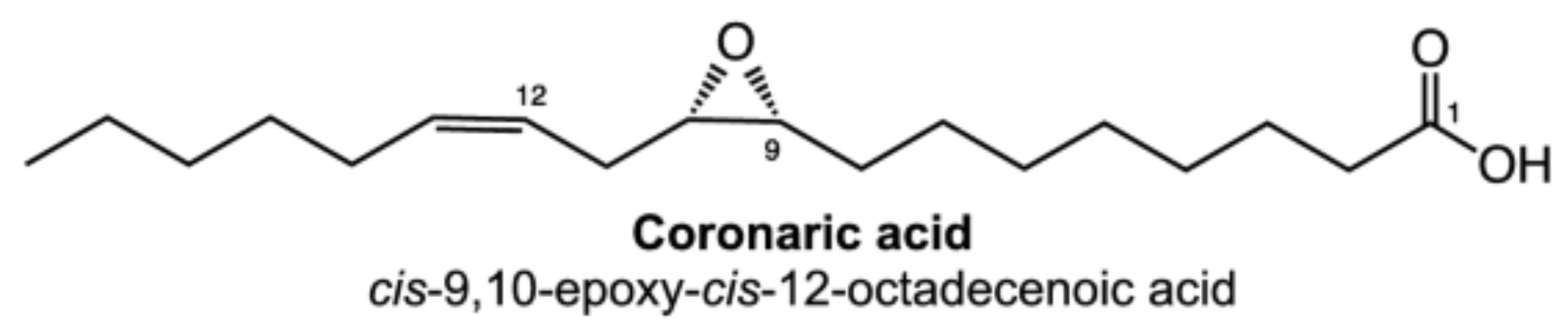
These two epoxy acids are commonly present in cutin and suberin up to an amount of 34% of the lipid content.
Vernonia and Stokesia species are among plants identified as containing epoxy fatty acids at high percentages in their seed oils. Works are in progress in Africa to improve crops of these plants.
Vernolic acid is the best known epoxy acid from natural sources. This isomer of coronaric acid is 12,13-epoxy-9-octadecenoic acid was characterized as an epoxy oleic acid (Gunstone FD, J Chem Soc 1954, 1611) and was shown to be abundant in seed oils of the Compositae Vernonia sp as well as Euphorbia lagascae (from 60 up to 75%).
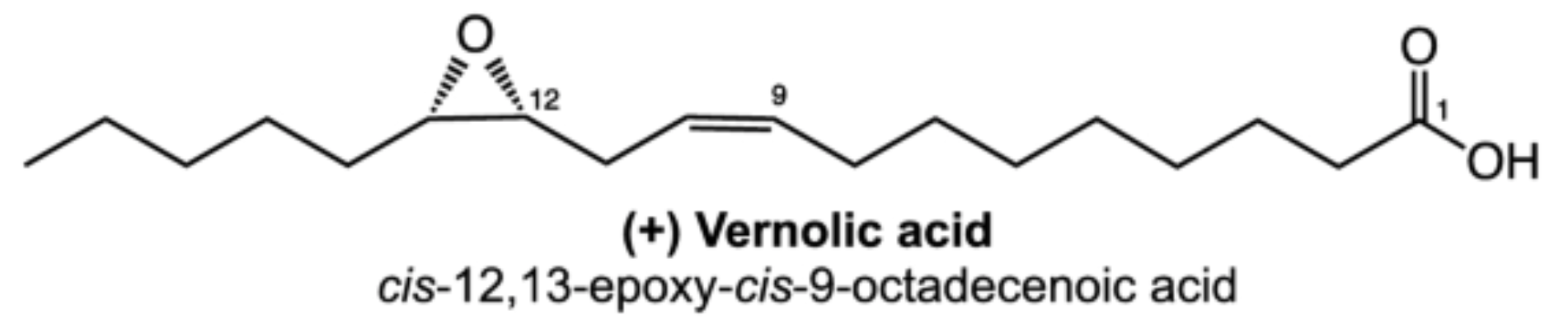
Small quantities (about 2.5%) of 9,10-epoxy-18:0, vernolic acid and coronaric acid were found in peanut (Arachis hypogaea) germplasm (Hammond EG et al., JAOCS, 1997, 74, 1235). Vernolic acid was also shown to be present (7%) in the seed oil of Geranium sanguineum (Geraniaceae) (Tsevegsuren N et al. Lipids 2004, 39, 571).
A C20 homologue of vernolic acid has been found in Alchornea cordifolia (Euphorbiaceae) seed oil (Kleiman R et al., Lipids 1977, 12, 610). This new fatty acid, cis-14,15-epoxy-cis-11-eicosenoic acid has been named as alchornic acid.
Epoxide groups are also present in bacterial lipids.
Epoxides may be also formed during peroxidation attack of unsaturated fatty acids. This chemical reaction (with peroxyformic acid) is used to transform vegetal oils (mainly canola) into bio-resins used in composite boards and in polyurethane adhesive and insulating foam applications (Kong X et al., Lipid Technol 2012, 24, 7).
In living organisms, coronaric and vernolic acids, known also as leukotoxins, were shown to be formed in lung, vascular system, and neutrophils by cytochrome P450 enzymes (epoxydases) interfering in the regulation of vascular tone, homeostasis, and blood pressure. Several studies suggested that these epoxides have toxic cardiovascular effects which may result in death at high doses. Their strong toxicity seems to be related to the disruption of the endothelial barrier function and a modulation of any dysfunction mediated by various contaminants (Slim R et al., Toxicol Appl Pharmacol 2001, 171, 184). Leukotoxins have been suggested to be a toxic mediator (“burn toxin”) causing acute respiratory distress syndrome in burn patients (Hayakawa M et al., Biochem Int 1990, 21, 573). Further studies have demonstrated that leukotoxin-diol, the hydrated product of leukotoxin, is even more toxic that the parent leukotoxin in vitro (Moghaddam MF et al., Nature Med, 1997, 3, 562) and in vivo (Zheng J et al., Am J Respir Cell Mol Biol 2001, 25, 434). Thus, these by-products are the putative toxic mediators involved in the development of acute respiratory distress syndrome.
Epoxides of arachidonic acid, EPA and DHA are also formed by cytochrome P450 and are very potent bioactive molecules.
Hydroxy derivatives of epoxy-arachidonic acid (hepoxilins) are described with the bioactive lipoxygenase products.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire