
Furan fatty acids : These fatty acids contain a furan ring in the chain. They were found for the first time in a seed oil of Exocarpus cupressiformis (Santalaceae) (Morris LJ et al., Tetrahedron Lett 1966, 36, 4249).
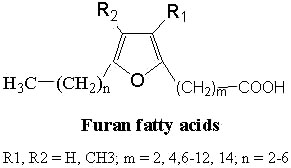
The furan acid described initially was the 9,12-epoxy-octadeca-9,11-dienoic acid (R1, R2 = H, m = 7 and n = 5 in the general formula above).
A common nomenclature describing these fatty acids (as F1, F2,…) is used. This naming originated from elution order in gas chromatography.
The most frequent compound is known as F6 (12,15-epoxy-13,14-dimethyleicosa-12,14-dienoic acid) while, later, two series of propyl- and pentyl-substituted F-acids in the 5-position of the furan ring was described in fish, crayfish, soft coral and various algae and terrestrial plants. Their presence was more recently detected in mammals including man (Puchta V et al., Liebigs Ann Chem 1988, 25). In man, furan fatty acids were located in phospholipid fractions. Studies have shown that a dibasic urofuran acid, 3-carboxy-4-methyl-5-propyl-2-furanopropanoic acid, is found in high concentrations in subjects diagnosed with prediabetics, gestational diabetes, and T2D (Liu Y et al., Cell Reports 2016, 14, 2889). An increase in its concentration has been recognized as a potential biomarker in diabetes development. 3-Carboxy-4-methyl-5-pentyl-2-furanopropanoic acid has also been identified in human plasma and urine (Prentice KJ et al., EBioMedicine. 2018; 27: 200).
In fish, furan fatty acids are found in liver cholesterol esters and in testis triglycerides, but are also present in phospholipids. In 1977, height furan fatty acids were listed in several organs of 20 fresh water fish species (Glass RL et al., Lipids 1977, 12, 828). Forty furan fatty acids were identified in a marine fish oil, two propyl-substituted molecules being reported for the first time (Wahl HG et al., J High Resol Chromatogr 1994, 17, 308).
As a furan fatty acid (10,13-epoxy-11-methyloctadeca-10, 12-dienoic acid) was detected in the cellular lipids of several marine bacteria (Shirasaka N et al., Biochim Biophys Acta 1995, 1258, 225), the authors propose that those detected in marine fish are derived from marine plants and/or intestinal bacteria of fishes.
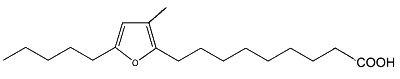
10,13-Epoxy-11-methyloctadeca-10, 12-dienoic acid
Three new furan derivatives (plakorsins A-C) were isolated from the Taiwanese marine sponge Plakortis simplex, n being equal to 15 and m being equal to 1. These fatty acids exhibited cytotoxic activity against cultured cells (Shen YC et al., J Nat Prod 2001, 64, 324). Furan acids were found in 10 genera of Gorgonaria corals without zooxanthellae (endosymbiotic microalgae of the genus Symbiodinium). Furan acids were not found in Gorgonaria with zooxanthellae, alcyonaria and other soft corals. A possible source of furan acids in Gorgonaria might have been microalgae occurring in food or bacteria and microalgae associated with the corals (Imbs AB et al., Chem Nat Compounds 2009, 45, 898).
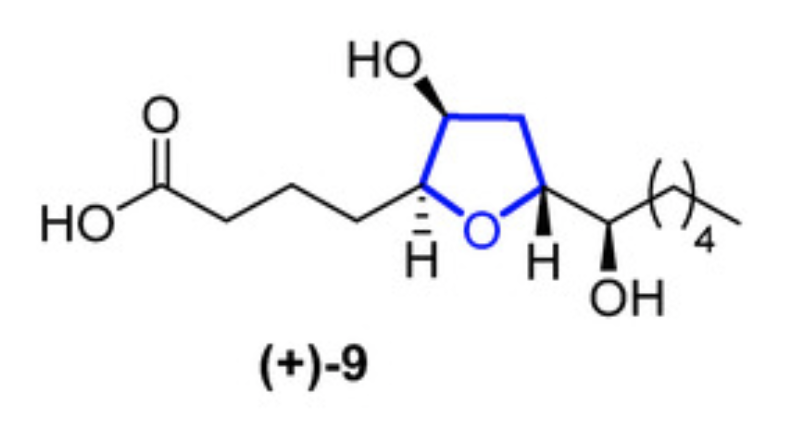
In 2015, Li reported the isolation of petromyroxols (Li K et al., Org. Lett. 2015, 17, 286). They are oxylipids isolated from water conditioned with larval sea lamprey Petromyzon marinus L. One of them, the (+)-9 isomer, shows a potent olfactory response of 0.01 to 1 μM in the sea lamprey.
Petromyric acids are dehydroxylated tetrahydrofuranyl fatty acids that were isolated from larval washing extracts from the sea lamprey Petromyzon marinus in 2018 (Li K et al., PNAS 2018, 115, 8603).
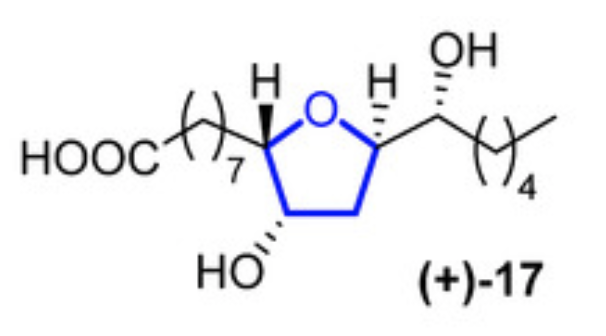
Sea lampreys are anadromous fishes that migrate, using their olfactory cues to orientate, from the ocean to freshwater to find a suitable spawning stream. When approaching river mouths, the decision of which stream is optimal to spawn in is taken using their olfactory system to detect a pheromone emitted from larval sea lampreys. When investigating larval washing extracts, four fatty acids were identified, but only (+)-17 petromyric acid has proven to be the pheromone that guides lamprey adults. Fatty acid analogues have been reported to be pheromones in insects, but this is the first identification in fish.
Due to the analytical difficulties in their determination furan fatty acids have been scarcely determined in food. There was an early report of the presence of a furan acid in latex rubber from Hevea brasiliensis (Hasma H et al., Lipids 1978, 13, 905). This was confirmed by the identification of a furan fatty acid (10,13- epoxy-11-methyl octadeca-10,12 dienoic acid) esterified in latex glycoglycerolipids (Liengprayoon S et al., Phytochemistry 2011, 72, 1902). Other vegetal sources were described. Thus, several furan species were found in grasses (240 mg/g dry w.), lemon (30 mg/g dry w.), strawberry, orange, potato (2-5 mg/g dry w.) and even in mushroom (160 mg/g dry w.) (Hannemann K et al., Lipids 1989, 24, 296). Furan fatty acids have been studied in asparagus (Asparagus officinalis) (Müller F et al., JAOCS 2024, 101, 383). The total amounts of furan fatty acids ranged from 1.4 to 4.6 mg/100 g dry weight. 22 different furan fatty acids-containing triacyglycerol have been detected. Accordingly, during the season white asparagus can serve as a valuable source for furan fatty acids and antioxidants, especially for vegetarians or vegans.
It has been described that 11-(3,4-dimethyl-5-penylfuran-2-yl)-undecanoic acid (11D5) is the prevalent furan fatty acid in tea matrix. 11D5 was detectable in all samples of green and black tea infusions (Romanotto A et al., JAOCS 2022, 99, 501). Amounts of 11D5 were higher in green tea than in black tea. Moreover, Darjeeling tea infusions were by ~30% richer in 11D5 than black and green teas from other regions. Each cup of green and black tea infusion may provide 20–60 μg 11D5, which is about 5% of the amounts found in tea samples.
By far the richest source of furan fatty acids in food is fish. Concentrations of up to 4 g/100 g oil were reported in the literature (Vetter W et al., J. AOCS 2012, 89, 1501). Frequently, the furan acid concentrations were in the range 0.1-0.9 g/100 g oil, with a clear dominance of dimethylated derivatives. Lower concentrations have been reported in other fatty sources : butter (48 mg/100 g), soybean oil (42 mg/100 g), wheat germ oil (22 mg/100 g).
An extensive review of the occurrence of furan fatty acids may be found (Spiteller G, Lipids 2005, 40, 755). A review of their importance in food may be also consulted (Vetter W et al., Lipid technol 2013, 25, 7). Furan fatty acids have been described in 37 commercial or collected edible and meadow fungi, their distribution and concentration are reported (Müller F et al., J Agric Food Chem 2022, 70, 12620).
It appears possible that these furan compounds may serve as antioxidants in biological systems through their ability to scavenge free radicals (Okada Y et al., Biol Pharm Bull 1996, 19, 1607). The radical scavenging Furan acids are able to eliminate radicals before their concentrations are exponentially increasing by means of a radical chain reaction. The strong capability of these compounds to serve as radical scavengers suggests that plants and algae can use these compounds to defend against the deleterious effect of UV radiation (Spiteller G, Lipids 2005, 40, 755). A review has focused mainly on the functions and roles of furan acids in plants (Mawlong I et al., Phytochem Rev 2016, 15, 121). It is recalled that In plants, they are bound to phospholipids by substituting polyunsaturated fatty acids and function as free radical scavengers suggesting their role in defense against oxidative stress.
Antifungal activity was associated with some natural furan fatty acids, such as 2-heptyl-5-hexylfuran-3-carboxylic acid, and reinforced considering some of their derivatives (Guo L et al., J Agric Food Chem 2025, 23 May).
It was hypothesized that the radical-scavenging ability of furan fatty acids could contribute to the protective properties of fish diets from heart disease (Spiteller G, Lipids 2005, 40, 755). A first in vivo demonstration has been given using furan fatty acids isolated from a mussel Perna canaliculus, native to the New Zealand coast (Wakimoto T. et al., PNAS 2011, 108, 17533). These fatty acids exhibited in a rat model of adjuvant-induced arthritis a more potent anti-inflammatory activity than that of EPA. These anti-inflammatory effects corroborate the observation of a lower incidence of arthritis in coastal Maoris, eating these mussels, compared with its prevalence in European or inland Maori people.
The emerging significance of furan fatty acids in food, nutrition, and potential therapeutic use has been extensively examined (Chang F et al., Food Chem 2025, 143759). The chemical synthesis of furan fatty acids was reviewed, paving the way for future animal and human studies. Furan fatty acids exhibit potent antioxidant and anti-inflammatory effects, with growing evidence of their role in metabolic health. Recent research suggests that they may extend benefits beyond omega-3 fatty acids in promoting cardiovascular and metabolic health.
A review exposed controversies and raised questions about whether furan fatty acids are markers for healthy diets or indeed associated with diabetes and renal health. Authors concluded that, on balance, furan fatty acids are beneficial for health (Xu L et al., Prog Lipid Res 2017, 68, 119-137).
A rapid method for the preparation of C18 furanoid fatty aster from methyl ricinoleate involving dry-column chromatography was described (Lie Ken Jie MSF et al., JAOCS 1981, p. 705).
A method for the quantitative determination of furan acids in food has been reported (Vetter W et al., JAOCS 2012, 89, 1501). After extraction, transesterification and silver ion chromatography, the furan acids are anayzed by GC/EI-MS.
Paraconic acids, also referred to as aliphatic lactones, are a compound group showing typical structural features consisting of a γ-butyrolactone ring bearing a carboxylic acid group, a long alkyl side chain, and either a methylene group or a methyl group. Among these, protolichesterinic acid is the best studied representative that has shown antibiotic, antiproliferative, and antiviral properties and can be isolated from the lichen Cetraria islandica, the only lichen species, which persisted in traditional European medicine when the use of lichens was abandoned during the 19th century (Ingolfsdottir K et al., Phytomedicine 1994, 1, 187). Paraconic acids display a broad range of biological activities such as antibiotic and antitumor properties. A review of the pharmacological activities and recent synthetic advances of γ-butyrolactones may be consulted (Our J et al., Int J Mol Sci 2021, 22, 2769).
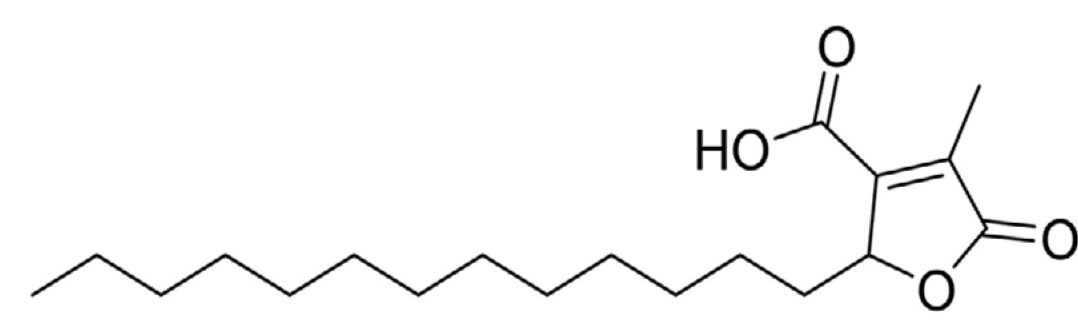 Protolichesterinic acid
Protolichesterinic acid
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire