
Short chain w-phenylalkanoic acids have long been known to occur in natural products.
The simplest one is benzoic acid. This acid is present, free or esterified, in several plants.
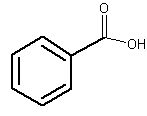
Benzoic acid
Appreciable amounts have been found in most berries (around 0.03 to 0.15%). Ripe fruits of several Vaccinium species (Ericaceae) (e.g., cranberry, Vaccinium vitis idaea; bilberry, Vaccinium macrocarpon) contain the highest concentration of free benzoic acid. Gum benzoin, a balsamic resin produced by shrubs (Styrax species) from tropical regions, mainly in Asia, contains up to 20% of benzoic acid and 40% benzoic acid esters. The name “benzoic” is derived from the name of these resins. In perfumery, benzoin is used as a fixative, slowing the dispersion of essential oils into the air.
Phenylacetic, 3-phenylpropanoic and 3-phenylpropenoic (cinnamic) acids are present in propolis, mammalian exocrine secretions or plant fragrances.
Cinnamic acid is obtained from cinnamon or from balsam. It is also found in shea butter. It is used as a component of several flavors and certain pharmaceuticals (as methyl, ethyl or benzyl esters).
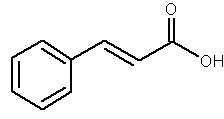
Cinnamic acid
Cinnamic acid is formed biosynthetically from phenylalanine and is metabolized through hydroxylation and methylation into various polyphenols with antioxidant properties (caffeic, coumaric, ferulic and sinapic acids). Considering their high polarity, these compounds cannot be considered as true lipids.
During a systematic study of the lipids from seeds of the plant Araceae, Schmid PC et al. (Phytochemistry 1997, 45, 1173) discovered the presence of 13-phenyltridecanoic acid as a major component (5-16% of total fatty acids). Other similar compounds but with 11 and 15 carbon chain lengths and saturated or unsaturated were shown to be also present but in lower amounts. At the same time, the even carbon chain w-phenylalkanoic acids of C10 up to C16 were discovered in halophilic bacteria (Caballeira NM et al., Lipids 1997, 32, 1271).
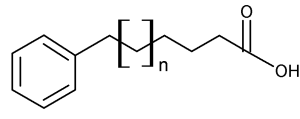
w-phenylalkanoic acid (x = 1 to 17)
Later, an exhaustive study of 17 genus of the subfamily Aroideae of Araceae revealed the presence of three major acids, 11-phenylundecanoic acid, 13-phenyltridecanoic acid and 15-phenylpentadecanoic acid in seed lipids (Meija J et al., Phytochemistry 2004, 65, 2229). Other odd carbon number acids from C7 to C23 were detected but in trace amounts. Similarly, two series of homologous odd carbon number monounsaturated w-phenylalkanoic acids were found.
Thus, it can be stated that all odd carbon chain w-phenylalkanoic acids from C1 through C23 have been found in nature. Furthermore, even carbon chain w-phenylalkanoic acids from C10 through C16 were also detected.
Two peroxidized isomeric phenylhexadecanoic acids, epiplakinic acids, have been isolated from the Palauan sponge Plakortis nigra (Sandler JS et al., J Nat Prod 2002, 65, 1258). One of them is shown below. Both acids inhibited the HCT-116 human colon tumor cell line.
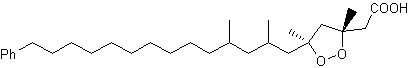
Epiplakinic acid
Substituted phenylalkenoic acids are periodically encountered in nature. As an example, rubrenoic acids were purified from Alteromonas rubra, compounds which showed bronchodilatatoric properties (Holland GS et al., Chem Ind 1984, 850).
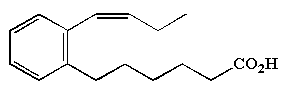
Methyl phenylalkenoic acids (5 carbon chain) have been described from a terrestrial Streptomycete (Mukku VJ et al., Z Naturforsch 2002, 57b, 335).
Serpentene, a similar polyunsaturated phenylalkenoic acid, is also produced by Streptomycesand was shown to have some antibacterial properties.
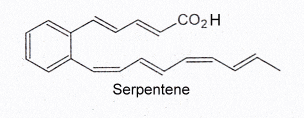
Several serpentene-like compounds have also been isolated from the same bacterial source (Wenzel SC et al., J Nat Prod 2004, 67, 1631).
Several bicyclic derivatives of linolenic acid were shown to be generated by alkali isomerization (Matikainen J et al., Tetrahedron Lett 2003, 59, 567).
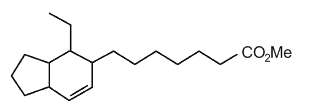
Bicyclic hexahydroindenoic acid
Some others (alkyl-phenyl)-alkanoic acids) are formed when linolenic acid is warmed at 260–270°C (Hase A et al., JAOCS 1978, 55, 407).
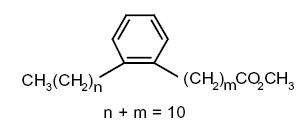
Several forms with 20 or 22 carbon atoms were identified in archaeological pottery vessels and were presumed to have been generated during heating of highly unsaturated fatty acids. They were used as biomarkers to trace the ancient processing of marine animal in these vessels (Craig OE et al., PNAS 2011, 108, 17910).
Several benzoic acid derivatives have been described in leaves of various Piperaceae species. Thus, a prenylated benzoic acid acid derivative, crassinervic acid, has been isolated from Piper crassinervium (Lago JH et al., J Nat Prod 2004, 67, 1783).
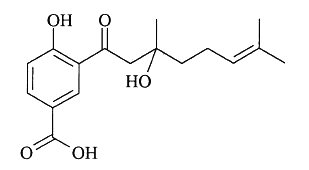
Crassinervic acid
Similar compounds were isolated from P. aduncum (aduncumene) and P. gaudichaudianum(gaudichaudianic acid). All these molecules showed high potential as antifungal compounds. A prenylated benzoic acid with a side chain formed of two isoprene units has also been isolated from the leaves of P. aduncum (Baldoqui DC et al., Phytochemistry 1999, 51, 899). More recently, three prenylated benzoic acid derivatives with four isoprene units have been extracted from the leaves of Piper heterophyllum and P. aduncum (Flores N et al., Phytochemistry 2009, 70, 621). These compounds displayed moderate antiplasmodial (against Plasmodium falciparum) and trypanocidal (against Trypanosoma cruzi) activities.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire