
They have commonly straight chains and even carbon number (4-30). They have the general formula: CH3(CH2)nCOOH
They are named from from the saturated hydrocarbon with the same number of carbon atoms, the final -e is changed to -oic. For example, the fatty acid with 18 carbon atoms is correctly termed octadecanoic acid but it has also a trivial name (as several common fatty acids), i.e. stearic acid. This compound may be defined also 18:0.
Below, is found a list of the most common saturated fatty acids.
|
Systematic name |
Trivial name |
Shorthand designation |
Molecular wt. |
Melting point (°C) |
| propanoic | propionic | 3:0 | 74.1 | -20.5 |
| butanoic | butyric | 4:0 | 88.1 | -7.9 |
| pentanoic | valeric | 5:0 | 102.1 | -34.5 |
| hexanoic | caproic | 6:0 | 116.1 | -3.4 |
| heptanoic | oenanthic | 7:0 | 130.1 | -7.5 |
| octanoic | caprylic | 8:0 | 144.2 | 16.7 |
| nonanoic | pelargonic | 9:0 | 158.2 | 12.5 |
| decanoic | capric | 10:0 | 172.3 | 31.6 |
| undecanoic | undecylic | 11:0 | 186.3 | 29.3 |
| dodecanoic | lauric | 12:0 | 200.3 | 44.2 |
| tetradecanoic | myristic | 14:0 | 228.4 | 53.9 |
| pentadecanoic | 15:0 | 242.4 | 51-53 | |
| hexadecanoic | palmitic | 16:0 | 256.4 | 63.1 |
| heptadecanoic | margaric (daturic) | 17:0 | 270.4 | 61.3 |
| octadecanoic | stearic | 18:0 | 284.4 | 69.6 |
| eicosanoic | arachidic | 20:0 | 312.5 | 75.3 |
| docosanoic | behenic | 22:0 | 340.5 | 79.9 |
| tetracosanoic | lignoceric | 24:0 | 368.6 | 84.2 |
| hexacosanoic | cerotic | 26:0 | 396.7 | 88 |
| heptacosanoic | carboceric | 27:0 | 410.7 | |
| octacosanoic | montanic | 28:0 | 424.8 | |
| triacontanoic | melissic | 30:0 | 452.9 | |
| dotriacontanoic | lacceroic | 32:0 | 481 | |
| tritriacontanoic | ceromelissic (psyllic) | 33:0 | 495 | |
| tetratriacontanoic | geddic | 34:0 | 509.1 | |
| pentatriacontanoic | ceroplastic | 35:0 | 523.1 |
SOLUTION PROPERTIES
Normal fatty acids exhibit appreciable solubility in water compared to the corresponding hydrocarbons due to the presence of the polar carboxyl group. The first members of the saturated fatty acid series are miscible with water in all proportions at room temperature.
Solubility in water at 20°C (in grams acid per liter)
| Carbon number | Solubility |
| 2 | infinite |
| 3 | infinite |
| 4 | infinite |
| 6 | 9.7 |
| 8 | 0.7 |
| 9 | 0.3 |
| 10 | 0.15 |
| 12 | 0.055 |
| 14 | 0.02 |
| 16 | 0.007 |
| 18 | 0.003 |
The solubility behavior of the fatty acids in organic solvents is of considerable theoretical and industrial importance. Solubility data for the most common saturated fatty acids are given in the table below (in grams per liter at 20°C).
| Carbon number | Chloroform | Benzene | Cyclohexane | Acetone |
Ethanol 95% |
Acetic acid | Methanol | Acetonitrile |
| 10 | 3260 | 3980 | 3420 | 4070 | 4400 | 5670 | 5100 | 660 |
| 12 | 830 | 936 | 680 | 605 | 912 | 818 | 1200 | 76 |
| 14 | 325 | 292 | 215 | 159 | 189 | 102 | 173 | 18 |
| 16 | 151 | 73 | 65 | 53.8 | 49.3 | 21.4 | 37 | 4 |
| 18 | 60 | 24.6 | 24 | 15.4 | 11.3 | 1.2 | 1 | <1 |
On the basis of solubility data, it can be concluded that the normal saturated fatty acids are generally more soluble in chloroform and less soluble in acetonitrile than in any of the organic solvents investigated.
![]()
Up to 6 (or 4) carbon atoms, organic acids are considered “short-chain organic acids”, they have substantial solubility in water. Furthermore, they do not behave physiologically like other fatty acids since they are more rapidly digested and absorbed in the intestinal tract and have unique properties in regulating sodium and water absorption through the mucosal epithelium. Biochemically, they are more closely related to carbohydrates than to fats. These short-chain fatty acids are metabolites of specific bacterial taxa of the animal gut microbiota, and, as far as we know, their production is influenced in human by specific foods or food supplements, mainly prebiotics. These compounds play a key role in health and disease, as they regulate gut homeostasis and their deficiency is involved in the pathogenesis of several disorders, including inflammatory bowel diseases, colorectal cancer, and cardiometabolic disorders (Fusco W et al., Nutrients 2023, 15, 2211). A review has highlighted the crucial role of short-chain fatty acidss in maintaining intestinal health, immune function, and their implications in various diseases (Abdelhalim KA et al., Chem-Biol Interact 2024, 388, 110851). It was shown that they are crucial in barrier integrity and immunity against viral infections (Feng C et al., Biomed Pharmacother 2023, 160, 114414). A review discussed the mechanistic understanding of the direct and indirect influence that short-chain fatty acids have on brain function, behaviour and physiology (O’Riordan KJ et al., Mol Cell Endocrinol 2022, 546, 111572).
From 7(or 6) to 12 carbon atoms, fatty acids are said to have a medium chain. Physiological studies have shown that ingestion of triglycerides containing these medium-chain fatty acids, including caprylic acid (C8:0) and capric acid (C10:0), which occur in milk fat, palm oil, and various feed materials, differ from long chain fatty acids in digestion, absorption, and metabolism, and studies have found that they can reduce body weight and improve internal fat accumulation and cholesterol metabolism. Thus, they facilitate weight control when included in the diet as a replacement for long-chain triglycerides (St-Onge MP et al., J Nutr 2002, 132, 329). It has been demonstrated that heptanoate is more effective in fueling the Krebs cycle than even-chain fatty acids such as octanoate and studies strongly suggests that triheptanoin is able to improve the brain metabolic profile of patients with huntington disease (Adanyeguh IM et al. , Neurology 2015, 84, 490). Further studies found that C8:0 could inhibit the levels of inflammatory cytokines (Zhang X et al., Nutrients, 2023, 15, 1296).
Fatty acids which have 14 and more carbon atoms are considered as long-chain fatty acids.The specific physiological roles of saturated fatty acids have been reviewed (Legrand et al., Eur J Lipid Sci Technol 2015, 117, 1489).
Fatty acids with 4 to 12 carbon atoms are found mainly in milk fats (mainly butyric acid in cow and decanoic acid in sheep) but those with 10 and 12 carbon atoms are found also in certain seed oils such as coconut and other kernel fats of the palm family. Nevertheless, bacterial fermentation of amylase resistant starch and nonstarch polysaccharides in the gut is probably the most important source of short-chain fatty acids in humans
and most mammalian species.
Fatty acids with odd-numbered straight chains (odd-chain FAs) are commonly found in nature; however, they account for only a small fraction, usually less than a few tenths of the total fatty acid content. Mammalian milk and dairy products (cheeses, yogurts, etc.) are exceptions. These fatty acids are produced by biosynthesis carried out by symbiotic bacteria in the digestive tract and by de novo biosynthesis in the mammary gland. Odd-chain triacylglycerols containing at least one odd-chain fatty acid, e.g. margaric or margaroleic acids, whose concentrations in TAGs did not exceed 1 % of total TAGs, were also identified in vegetable oils (Holčapek M et al., J Chromatogr A, 2003, 1010, 195).
It has been suggested that 15:0 and 17:0 from adipose tissue or plasma could be considered as potentiel biomarkers of milk products intake (Wolk A et al., J Nutr 2001, 131, 828). It has been shown that the same relationship can be established with saturated fatty acids (15:0, 17:0, 20:0) acylating lysophosphatidylcholine (Nestel PJ et al., Am J Clin Nutr 2014, 99, 46). However, these odd-chain fatty acids can also be synthesized endogenously, for example, from gut-derived propionic acid (3:0). A number of studies have also shown an inverse association between the concentrations of these compounds in human plasma phospholipids or erythrocytes and risk of type 2 diabetes and cardiovascular disease (Pfeuffer M et al., Adv Nutr 2016, 7, 730).
It was proposed a possible involvement in metabolic regulation from the assumption that there is a link between 15:0 and 17:0 and the metabolism of other short-chain, medium-chain, and longer-chain odd-chain fatty acids (Pfeuffer M et al., Adv Nutr 2016, 7, 730).
Epidemiological studies have shown that higher circulating levels of odd-chain saturated fatty acids (C15:0 and C17:0) are associated with lower risk of metabolic disease. These fatty acids are produced by α-oxidation in peroxisomes, de novo lipogenesis, from the diet and by gut microbiota. Although present at low concentrations, they are of interest as potential targets to reduce metabolic disease risk (review in: Ampong I et al., Int J Biochem Cell Biol 2022, 143, 106135).
C15:0 is able to activate AMPK and inhibit mTOR, both of which are core components of the human longevity pathway. Experimental research support C15:0 as an essential nutrient with activities equivalent to, or surpassing, leading longevity-enhancing candidate compounds (Venn-Watson S et al., Nutrients, 2023, 15, 4607). Further, C15:0 has antimicrobial properties, including growth inhibition of pathogenic bacteria and fungi (Galdiero E et al., Res Microbiol 2021, 172, 103880 ; Ricciardelli A et al., Pathog Dis 2020, 78, ftaa012). Combined, these broad positive activities of C15:0 support its role as an essential nutrient to support long-term physiological health.
It has been demonstrated that supplementation of C15:0 at weaning in EFA-deficient rats increased early growth rate as also did the supplementation of C18:2 n-6. Furthermore, the supplementation of C15:0 in the diet of EFA-deficient animals induced the previously undescribed synthesis of odd-chain ppolyunsaturated fatty acids of the n-8 family (C19:3, C21:3 and C21:4 n-8). These results suggest dietary C15:0 might counteract EFA induced growth retardation, possibly through the synthesis of odd-chain n-8 PUFAs (Ciesielski V et al., J Nutr Biochem 2025, 137, 109814).
Studies have revealed that bottlenose dolphins with higher circulating concentrations of odd-chain saturated fatty acids (C15:0 and C17:0) have a lower risk of having metabolic syndrome and related conditions; further, increasing dolphins’ dietary odd-chain fatty acids resulted in improved cardiometabolic, iron, and red blood cell indices. In the field of cognition, it was assessed potential correlations between plasma fatty acid levels and cognitive test scores among adult patients with type 2 diabetes (Shen J et al., Diabetes Metab Syndr Obes 2022, 15, 1423). This study showed that people with higher C15:0 concentrations had higher total Mini-Mental State Examination (MMSE) scores, including higher MMSE orientation force scores. People with higher C15:0 concentrations also had higher total Montreal Cognitive Assessment (MoCA) scores as well as higher visual-spatial ability scores. It was further demonstrated that aging-associated amyloid-β plaques and neuroinflammation in bottlenose dolphins (Tursiops truncatus) were supporting roles of C15:0 (Venn-Watson S et al., Int J Mol Sci 2025, 26(8), 3746). These findings suggest that, in addition to protecting against Altzeimer’s disease co-morbidities, C15:0 may play a distinct role in supporting cognitive health, especially at higher concentrations.
It has been suggested that C26:0 may constitute a convenient blood biomarker of dementia that could be useful in routine medical practice (Zarrouk A et al., J Alzheimers Dis 2015, 44, 1349). This could be the result of an alteration of fatty acid metabolism in demented patients and points toward possible peroxisomal dysfunction.
A review has evaluated potential roles of odd-chain fatty acids in mitigating chronic diseases supporting the hypothesis for odd chain molecules as essential dietary lipids (Dornan K et al., JAOCS 2021, 98, 813-824).
Some peculiarities of the metabolic features of short-chain and medium-chain fatty acids that differ from those of long-chain fatty acids are summed up in an important review (Schonfeld P et al., J Lipid Res 2016, 57, 943).
Propionic acid (3:0) is not found in fats but is produced in the intestinal lumen as an end product of the fermentation of dietary fibers of anaerobic microbiota (den Besten G et al., J Lipid Res 2013, 54, 2325). It is the smallest organic acid that exhibits the properties of the other fatty acids, such as producing an oily layer when salted out of water and having a soapy potassium salt.
It has been shown to exert multiple beneficial effects on mammalian energy metabolism and also contributes to sending a signal to the brain that participate to the control of the liver neoglucogenesis.
The current literature suggests that under certain conditions, excess levels of propionate may play a role in Alzheimer’s disease. The cause of the excessive levels of propionate may be related to the Bacteroidetes phylum, which are the primary producers of propionate in the human gut (review in: Killingsworth J et al., Front Aging Neurosci 2021, 12:580001). Experiments in mouse models of Alzheimer’s disease have shown that administration of sodium propionate improved cognitive and memory function (Lang W et al., Neurosci Lett 2022, 791:136887). An exploration of the gut–brain axis in human has demonstrated an association between increased circulating propionic acid levels and higher cognitive decline in older persons (Neuffer J et al., Nutrients 2022, 14, 4688). Propionic acid may be derived from the fermentation of undigested dietary fiber by the gut microbiota, as well as from dietary intake, as it is a common food preservative in processed food.
Butyric acid (4:0) is the lowest member of the acetic acid series found in natural fats. It occurs (2 to 4%) as a component of milk fats. It gives a rancid odor to butter when triglycerides are hydrolyzed and is present in fermentation products of carbohydrates. Butyric acid is commonly used in pharmacological studies as it is a well-known histone deacetylase inhibitor that results in increased histone acetylation when applied to cells in culture in the high micromolar range. Numerous observations are consistent with the idea that butyrate can modulate the expression of a large number of genes to affect numerous pathophysiological pathways even in the brain (Bourassa MW et al., Neurosci Lett 2016, 625, 56). This fatty acid has peculiar physiological properties in causing growth arrest and apoptosis in various cell types (Urbano A et al., Leukemia 1998, 12, 930). It was tested in the therapy of solid tumors or leukemia (Kasukabe T et al., Br J Cancer 1997, 75, 850). It was also shown to be a chemical factor capable of promoting pluripotent stem cell generation (Liang G et al., J Biol Chem 2010, 285, 25516). With propionic acid, butyric acid presents multiple effects in different cells involved in the inflammatory and immune responses (Vinolo M et al., Nutrients 2011, 3, 858). These fatty acids not only affect the function of leukocytes but can also induce apoptosis in lymphocytes, macrophages and neutrophils.
ß-Hydroxy ß-methylbutyric acid is a naturally produced substance in humans (a metabolite of leucine) that is currently used used as dietary supplement to reduce the loss of lean body mass (Wilson GJ et al., Nutr Metab 2008;5:1) and as an ingredient in some medical food products. It has also been proposed that it could improve working memory and cognitive flexibility in aging (Hankosky ER et al., Nutr Neurosci 2016).
A derivative containing selenium, 2-hydroxy-(4-methylseleno)butanoic acid, is used in animal nutrition to support selenoprotein synthesis and protect tissues against oxidative stress.
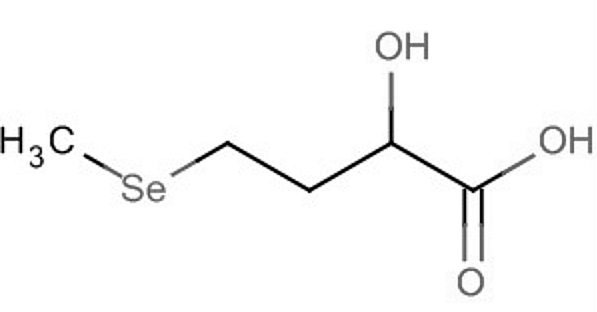
2-Hydroxy-(4-methylseleno)butanoic acid
Valeric acid (5:0) has been identified in petroleum distillates and in oxidation products of oils and fats and fermentation of carbohydrates. It has a putrid odor.
Caproic acid (6:0) occurs in milk fats to the extent of about 2%. It was first isolated from butter in 1816 by Chevreul. It has a characteristic odor of goats, hence its name (from the Latin caper, goat). Caproic acid is present as glucose ester in leaf trichomes of Datura metel.
Heptanoic acid (also called enanthic or oenanthic acid,is a colorless oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. Its salts and esters are called enanthates or heptanoates. The methyl ester of ricinoleic acid, from castor bean oil, is the chemical precursor of enanthic acid. used in fragrances, as an artificial flavors, in cosmetics, industrial lubricants and corrosion inhibitors. Enanthic acid is also used to esterify steroids in the preparation of drugs such as testosterone enanthate, Triheptanoin (triglyceride with three heptanoic acid molecules) is hydrolyzed in the small intestine. The heptanoic acid then enters β-oxidation to produce acetyl-CoA and propionyl-CoA that enters the citric acid cycle. In the liver, pentanoyl-CoA can serve as an anaplerotic substrate and generates 5-carbon ketone bodies (β-hydroxypentanoate and β-ketopentanoate), which can be utilized by peripheral tissues (Deng S et al., J Biol Chem 2009, 284, 27799).
Caprylic acid (8:0) is widely distributed in animal and vegetable fats but rarely exceeding 8% of the total fatty acids, except in the seed oils of two Lythraceae, Cuphea hookerina and C. painteri, which contain about 70% caprylic acid (Miller RW et al., JAOCS 1964, 41, 279). It occurs to an extent of 1 to 4% in milk fats, and 6 to 8% in coconut and palm oils. Caprylic acid is a component of the active form of ghrelin, a 28 amino acids peptide produced by the mammalian stomach (Kojima M et al., Nature 1999, 402, 656). Ghrelin binds to the growth hormone secretagogue receptor (GHSR-1a) located in the pituitary gland and hypothalamus and regulates many relevant biological processes including the secretion of growth hormone (GH), stimulation of appetite, food intake and modulation of gastric acid secretion and motility (Delporte, C., Scientifica 2013, 2013, 518909).
As in ghrelin, octanoylation concerns the formation of an ester bond between caprylic acid and the side chain of a serine residue (Ezanno H et al., Nutr Clin Metabol 2013, 27, 10).
Perfluoro-octanoic acid, a newly emerging persistent organic pollutant, is widely present in various environmental media. Reports have proved that this compound exposure can accumulate in the ovary and lead to reproductive toxicity (Zhang H et al., Int J Mol Sci 2024, 25, 136).
Pelargonic acid (9:0) is the first example of the occurrence of an odd-numbered carbon fatty acid in natural products. It occurs in secretion of sebaceous glands and as esters in essential oil of Pelargonium roseum from which it derives its name. It is also a primary product of oxidative fission of oleic acid. Nonanoic acid is also used in the preparation of plasticizers and lacquers. Its ammonium salt is an herbicide, commonly used in conjunction with glyphosate, a non-selective herbicide. It is a broad-spectrum contact bio-herbicide for the control of annual weeds. It destroys cell membranes, causing a rapid loss of cellular functions. Other saturated fatty acids, C6 or C14 long, are significantly less active than medium-chain acids, or have any herbicidal activity. Pelargonic acid is considered to have low toxicity and low environmental impact, and without residual activity. The Food and Drug Administration (FDA) has approved pelargonic acid as a food additive, and as an ingredient in solutions used commercially to peel fruits and vegetables.
Furthermore, it was shown effective in controlling seizure in association with a ketogenic diet (Chang P et al., Neuropharmacology 2013, 69, 105).
Capric acid (10:0) occurs as a minor component in the same fats that contain caprylic acid but also in the head oil of the sperm whale, and in wool and hair fats. It is a major constituent of elm seed oil (over 60% in Ulmus americana and over 70% in Zelkova serrata ) but is absent in other Ulmaceae (Apanthe, Morus) (Badami RC et al., Prog Lipid Res 1981, 19, 119). Similarly, it was discovered that the seed oil of a Lythraceae, Cuphea llavea, contained about 80% of this acid (Earle FR et al., JAOCS 1960, 37, 440).
Lauric acid (12:0) is one of the three most widely distributed saturated fatty acids found in nature (14:0, 16:0, and 18:0). It occurs extensively in Lauraceae seeds (Laurus nobilis) where it was discovered (Marsson T Ann 1842, 41, 329). It is dominant in cinnamon oil (80-90%), coconut oil (40-60% as trilaurin) and is found also in Cuphea species (Umbelliferae) whose production was initiated in Germany. The recent uses of lauric acid are in the manufacture of flavourings, cocoa butter, margarine, alkyd resins, soaps, shampoos and other surface active agents, including special lubricants. Lauric acid in its sulfated form, is an ingredient found in many household products (toothpaste, shampoos, shaving foams, and bubble baths).
Lauric acid as monoglyceride is known to the pharmaceutical industry for its good antimicrobial properties. It may play a role in combating lipid-coated RNA and DNA viruses. The major sources of lauric acid for human food are palm kernel, coconut and palm.
It has been demonstrated that lauric acid is the most effective in inhibiting 5α-reductase present in the prostatic epithelium, this effect being associated with a reduction of prostate hypertrophy by saw palm tree (Serenoa repens) extracts (Kwon Y, Food Sci Biotechnol 2019, 28, 1599).
Myristic acid (14:0) is present in major amounts in seeds of the family Myristicaceae (nutmeg oil – or oil of mace – from Myristica fragrans contains about 60-70% of trimyristin) where it was first discovered (Playfair L Ann 1841, 37, 152). Nutmeg is found in Moluccas and spice islands of Indonesia. Coconut and palm kernel are also convenient sources of 14:0 (trimyristine) which may be isolated in a pure form by distillation. It is also present in milk fats (8-12%) and in the head oil of the sperm whale (15%). An excess of myristic acid in the diet induces a rise in plasma cholesterol in animals and human being (Mensink RP et al., Arterioscler Thromb 1992, 12, 911). Among saturated fatty acids, only myristic acid is able to make an amide link with some cellular proteins (myristoylation), modification which regulates their biological activities (Johnson DR et al., Annu Rev Biochem 1994, 63, 869).
Pentadecanoic acid (15:0) or pentadecylic acid is an odd-chain fatty acid found mainly in diary products (milk, butter, cheese) as well as in ruminant meat. It has been found in higher concentrations in blood of people consuming these animal products and it was associated with a lower risk of developing type 2 diabetes (Imamura F et al., PLOS Medicine 2018, 15, e1002670 ) and cardiovascular disease (Trieu K et al., PLOS Medicine 2021, 18, e1003763).
Researchers in San Diego have shown 15:0 as an active dietary fatty acid that attenuates inflammation, anemia, dyslipidemia, and fibrosis in vivo, potentially by binding to key metabolic regulators and repairing mitochondrial function. Consequently, they proposed that, displaying broad health benefits, it could be considered as an essential fatty acid joining omega-3 and omega-6, the only fatty acids described as essential by nutrition scientists (Venn-Watson S et al., Scientific Reports 2020, 10, 8161). While more research is needed, these findings are promising.
Palmitic acid (16:0) is the commonest saturated fatty acids in plant and animal lipids.
 Palmitic acid
Palmitic acid
It has been purified first by Chevreul in his researches on butter and tallow, but was first surely characterized by Fremy E (Ann 1840, 36, 44), who prepared it in pure form from palm oil, from which he named it. Despite its wide distribution, it is generally not present in fats in very large proportions. It usually forms less than 5% of the total fatty acids, sometimes as much as 10% in common vegetal oils (peanut, soybean, corn, coconut) and in marine-animal oils. Lard, tallow, cocoa butter palm oil contain 25 to 40% of this component.
As for myristoylation, palmitoylation (S-acylation) corresponds to the reversible attachment of palmitic acid to the side chain of a cysteine residue via a thioester bond.
Free palmitic acid was shown to trigger early postembryonic development in starved Caenorhabditis elegans by suppressing intestinal mTORC1, a central hub of nutrient-sensing machinery (Ruan M et al., PLoS Biol 2024, 22, e3002841).
Stearic acid (18:0) was described by Chevreul (1823) in the course of his researches on fats. It is the highest molecular weight saturated fatty acid occurring abundantly in fats and oils. It occurs in small quantities in seed and marine oils. Milk fats (5-15%), lard (10%), tallow (15-30%), cocoa and shea butters ((30-35%) are the richest sources of stearic acid. It is the principal constituent of hydrogenated fats and oils (about 90%).
As all saturated fatty acids have been shown to contribute to increase levels of serum cholesterol, several indices have been proposed to assess the impact of diet on cardiovascular health (Chen J et al., Int J Mol Sci. 2020 Aug; 21(16): 5695).
The index of atherogenicity (IA) characterizes the atherogenic potential of fatty acids. As the PUFA/SFA ratio is too general and unsuitable for assessing the atherogenicity of foods, Ulbritcht and al. proposed a new index, IA, based on PUFA/SFA. The formula for calculating IA is:
IA =[C12:0+(4 × C14:0)+ C16:0] / Σ UFA
The IA indicates the relationship between the sum of SFAs and the sum of unsaturated fatty acids (UFAs). The main classes of SFAs, which include C12:0, C14:0, and C16:0, with the exception of C18:0, are considered pro-atherogenic. UFAs are considered to be anti-atherogenic as they inhibit the accumulation of plaque and reduce the levels of phospholipids, cholesterol, and esterified fatty acids.
The index of thrombogenicity (IT) was developed by Ulbritcht and al. in 1991. The formula is:
IT =(C14:0+ C16:0+ C18:0)/[(0.5× ΣMUFA)+(0.5×Σn−6 PUFA)+(3×Σn−3 PUFA)+(n−3 / n−6)]
The IT characterizes the thrombogenic potential of fatty acids, indicating the tendency to form clots in blood vessels and provides the contribution of different fatty acids, which denotes the relationship between the pro-thrombogenic fatty acids (C12:0, C14:0, and C16:0) and the anti-thrombogenic fatty acids (MUFAs and the n-3 and n-6 families)
![]()
The longer chains are less frequent, they can be found in uncommon seed oils (C20-24 in Leguminoseae and Sapindaceae), in palm oil (C20-C32)(Puah CW et al., Lipids 2006, 41, 305), in waxes (C24-30) and in some sphingolipids (C20-24). Long-chain saturated fatty acids (from C24 to C28) are produced by microalgae and it was estimated that diatoms contribute from 30 to 80% of these components in sandy sediments (Volkman JK et al., Org Geochem 1998, 29, 1163). These long-chain fatty acids derive from higher plant waxes and are more abundant in deep than in surface sediments (Rieley G et al., Org Geochem 1991, 17, 901; Muri G et al., Org Geochem 2004, 35, 1083).
Arachidic acid (20:0) occurs in appreciable quantities in groundnut (Arachis hypogea) oil (3%) where it was discovered in 1854 by Gössmann A (Ann Chemie 1854, 89, 1). Larger amounts are found in seeds of Sapindaceae (up to 20%). It is also found in the depot fat of some animals and in milk fats.
Behenic acid (22:0) was first reported as a constituent of ben (behen) oil (seeds of Moringa oleifera) (Voelcker A Ann 1848, 64, 342). Except for the seed oils of the Crucifereae (between 0.5 and 3.4%), this fatty chain does not occur in the principal oils. Large amounts are found in hydrogenated animal and vegetal oils (8-57%).
Lignoceric acid (24:0) is present at trace levels in plant oils except in groundnut oil (about 1%) and notably in a Leguminous seed oil (Adenanthera pavonina) where it may amount to about 25%. It is the principal fatty acid present in carnauba wax (30% of the normal fatty acids). A major source is rice-wax bran (about 40%).
Without double bonds or other functional groups, these fatty acids are nearly chemically inert and thus can be subjected to drastic chemical conditions (temperature, oxidation).
![]()
Saturated fatty acids were shown to be the major constituents of adipocere (similar to “adipocire” studied by Chevreul), the white and soap-like decomposition product which forms due to the post-mortem conversion of body adipose tissue (Pfeiffer S et al., J Forensic Sci 1998, 43, 368). Immediately following death, triglycerides are hydrolyzed into free fatty acids and glycerol. The free fatty acids (mainly myristic, palmitic, and stearic acids) present in characteristic relations (Forbes SL et al., For Sci Int 2002, 127, 225 ; Eur J Lipid Sci Technol 2003, 105, 761) are formed by hydrogenation of triglyceride components under suitable environmental conditions. During that conversion process a number of byproducts may be formed, such as hydroxy or keto fatty acid derivatives (Takatori T, For Sci Int 1996, 80, 49). The occurrence of salts of these saturated fatty acids has been suggested as resulting from reaction with the surrounding mineral environment.
Olfaction-based research have shown that carboxylic acids (from C4 to C16) play an essential role in the host-seeking behavior of the malaria mosquito Anopheles gambiae(Smallegange RC et al., J Chem Ecol 2009, 35, 933).
Saturated fatty acids with straight chain have been found in a number of sediments ranging in age from Precambrian to Recent. In most sediments, fatty acids with even-carbon chain are more abundant than those with odd-carbon chain. All fatty acids from C8 to C28 have been found in sediments (Kvenvolden KA, JAOCS 1967, 44, 628). Experiments suggest that normal paraffins in petroleum may be produced from normal fatty acids of longer chain lengths by decarboxylation or other chemical reactions.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire