
These compounds form the simplest form of lipids, they contain only carbon and hydrogen. They may be divided into aliphatic hydrocarbons with a carbon chain which may be linear (normal), branched, saturated (alkanes) or unsaturated (alcenes, alkynes), cyclic hydrocarbons and carotenoids. Several hydrocarbons may be substituted with oxygen-containing groups and may contain oxygen or nitrogen in one of their carbon rings.
These organic compounds include :
![]()
ALKANES – ALKENES – These molecules are found mainly in petroleum but living organisms, eukaryotic or prokaryotic, contain frequently hydrocarbons which are directly derived from fatty acids. They are known to be present in living matter since 1892 when Shall C (Chem Ber 1892, 25, 1489) identified undecane in ants, and Etard A (C R Acad Sci Paris 1892, 114, 364) identified eicosane in Bryonia dioica.
Those found in petroleum (and in the environment) are considered as mineral oil hydrocarbons or mineral oil products containing 10 to about 50 carbon atoms. Crude mineral oils are by far the predominant source of these compounds, but equivalent products can be synthesized from coal, natural gas or biomass. Mineral oils are complex mixtures of hydrocarbons, principally of straight and branched open-chain alkanes (paraffins) but also largely alkylated cycloalkanes (naphthenes) and aromatic hydrocarbons including mainly alkyl-substituted poly aromatic hydrocarbons.
Decane (C10H22), undecane (C11H24), and dodecane (C12H26) were detected in a Martian sample at the tens of pmol level and may originate from long-chain carboxylic acids. The provenance and distribution of these molecules are of high interest in the search for potential biosignatures on Mars (Freissinet C et al., PNAS, March 24, 2025). Numerous works have demonstrated that molecular features (16 for fatty acids, 14 for acyclic hydrocarbons) can potentially indicate sample origin. Terrestrial lipids are dominated by longer straight-chain molecules (C4-C34 fatty acids, C14-C46 acyclic hydrocarbons), with predominance for specific branched and unsaturated isomers. Lipid-like meteoritic soluble organics are shorter, with random configurations (Buckner DK et al., Astrobiology 2024, 24, 12 January).
Hydrocarbons derived from fatty acids (i.e. saturated alkanes and unsaturated alkenes) are ubiquitous in plants and insects where they often represent a major part of cuticular waxes and play an essential role in preventing water loss from the organisms to the dry terrestrial environment. In several insects, specific cuticular alkenes also act as sex pheromones. Occurrence of alkanes or alkenes has also been reported in various microorganisms (Ladygina L et al., Process Biochem 2006, 41, 1001). For example, synthesis of hydrocarbons is widespread in cyanobacteria (Coates RC et al., PLoS One 2014, 9:e85140), and it is thought that cyanobacterial alka(e)nes contribute significantly to the hydrocarbon cycle of the upper ocean. It has been demonstrted that several microalgae have the ability to convert C16and C18 fatty acids into alkanes and alkenes by a new, light-dependent pathway (Sorigué D et al., Plant Physiol 2016, 171, 2393). The decarboxylation of fatty acids is initiated through electron abstraction from the fatty acid by the photoexcited FAD with a quantum yield >80%, the enzyme complex was named fatty acid photodecarboxylase, thus it may be useful in light-driven, bio-based production of hydrocarbons (Sorigué D et al., Science 2017, 357(6354):903). Alkanes and alkenes of various chain lengths are important targets for biotechnology as they are major components of gasoline (mainly C5-C9 hydrocarbons), jet fuels (C5-C16), and diesel fuels (C12-C20).
These lipids are distinct from the terpenoid hydrocarbons. They have usually a straight chain of up to about 36 carbon atoms but may also be branched, with one or more methyl groups attached at almost any point of the chain. Usually, the methyl group is near the end of the chain (iso or anteiso). They are either saturated or unsaturated (mono or diunsaturated). In contrast with the diversity of methyl-branched alkanes found in insect species, n-alkanes predominate in plants. Among the least polar components of plant surface lipids hydrocarbons with the odd number carbon chains (C15 up to C33) are predominant.
Allenic hydrocarbons, such as 9,10-tricosadiene, 9,10-pentacosadiene, and 9,10-heptacosadiene were isolated from Australian insects (melolonthine scarab beetles) (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
Many microalgae contain the highly unsaturated alkene n-C21:6 formed by decarboxylation of the 22:6n-3 fatty acid (Lee RF et al., Phytochemistry 1971, 10, 593). A few species also contain the n-C21:5 alkene (Volkman et al., Org Geochem 1994, 21, 407). Several microalgae were shown to contain long-chain unsaturated alkenes from 19 to 38 carbon atoms and one to four double bonds (review in Volkman JK et al., Org Geochem 1998, 29, 1163).
Diverse mono-, di-, and tri-unsaturated alkenes were detected in eight lichen species isolated in Japan. It was found that the six lichens containing green algal photobionts were distinguished by the presence of 1,8-heptadecadiene, 6,9-heptadecadiene, and 8- and 7-heptadecenes. On the other hand, 1-octadecene, 4-octadecene, and 5-nonadecene were the major alkene components of the two lichens with cyanobacterial photobionts (Ikeda MA et al., Phytochemistry 2021, 189, 112823). These differences in characteristics could be attributed to phylogenetic differences in the photobionts that comprised the lichens, indicating that the alkene composition could be used for lichen chemotaxonomy (Ikeda MA et al., Organic Geochemistry 2023, 179, 104588).
Hydrocarbons are found at the outer surface in higher plant leaves. As an example, C27, C29, and C31 n-alkanes are the most abundant (from 11 to 19%) in needle wax of the Pinaceae Picea omorika (Nikolic B et al., Chem Nat Compounds 2009, 45, 697). In general, long chain n-alkane abundances (i.e. n-C25 and above) are typically higher in angiosperm trees and shrubs than in many gymnosperms, and more specifically, conifers (except Araucariaceae, Podocarpaceae, Taxaceae, and the Cupressoideae and Callitroideae groups within Cupressaceae which have n-alkane concentrations similar to those of angiosperms). The factors that influence the concentration of plant biomarkers and their carbon isotope composition and their use to understand climate, ecosystem, and carbon cycling in the geologic past have been reviewed (Diefendorf AF et al., Org Geochem 2017, 103, 1).
Environmental parameters such as temperature and humidity can affect the composition of higher plant leaf hydrocarbon wax. The abundance and distribution of long-chain n-alkanes in sediments have been proposed as chemical indicators reflecting climate change. Thus, it has been shown that aridity specifically affected the concentration and distribution of n-alkanes in Acacia and Eucalyptus sampled in the North of Australia. Their n-alkane concentration increased by a factor of ten from the sea to the dry center of Australia, but Acacia-alkanes decreased in average chain length towards the arid center of Australia, whereas Eucalyptus average chain length increased under arid conditions. (Hoffmann B et al., Org Geochem 2013, 62, 62).
Field bioassays enabled the discovery of alkanes as bee sex pheromones and orchid attractants. Thus, C21 to C27 alkanes elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37). The contribution of hydrocarbon to orchid pollination has been reviewed (Perkins J et al., Nat Prod Rep 2023,40, 819).
Its has been demonstrated that in the honey bee, the colony-specific cuticular hydrocarbon profile completes its maturation in foragers via a sequence of stereotypic age-dependent quantitative and qualitative chemical transitions, which are driven by environmentally-sensitive intrinsic biosynthetic pathways (Vernier CL et al., Elife 2019, 8:e41855). Later, authors were showing that differences in the chemical profiles of individuals from different social insect colonies are primarily driven by community-level variations in microbiota across colonies (Vernier CL et al., Sci Adv 2020, 6/42, eabd3431).
They are also abundant at the outer surface of insects and several marine organism. They are thought to serve as a barrier to water influx in the organism, to act as sex attractants (or anti-aphrodisiacs), to affect the absorption of chemicals and microorganisms. Wild populations of Drosophila melanogaster use several cuticular hydrocarbons (mainly 7,11-heptacosene) as sexual pheromone (Cobb M et al., Anim Behav 1990, 39, 1058). The roles of hydrocarbons in the recognition systems of insects has been reviewed (Singer TL, Amer Zool 1998, 38, 394). The blend of linear and branched hydrocarbons from 22 to 34 carbon atoms found on the cuticle of various species of the Coleoptera genus Chrysochus has been shown to mediate mate choice and sexual isolation (Petereson MA et al., Chemoecology 2007, 17, 87). The 7-methyltricosane is a male-predominant cuticular hydrocarbon used as a contact pheromone in the western flower thrips Frankliniella occidentalis (Thysanoptera) (Olaniran OA et al., J Chem Ecol 2013, 39, 559). 5-Methylheptadecane has been identified as the sex pheromone of Leucoptera spartifoliella appearing as a highly specific biological control agent for the Scotch broom, Cytisus scoparius (El-Sayed AM et al., J Chem Ecol 2024, 50, 874).
Several hydrocarbons are produced as alarm pheromones by the Dufour’s gland in the ants (Regnier F E et al., J Insect Physiol 1968, 14, 955). Five hydrocarbons have been described, undecane, tridecane, pentadecane, 2-tridecanone, 2-pentadecanone. During the act of stinging, formic acid and hydrocarbons are discharged simultaneously from these glands in fine droplets. These hydrocarbons act also as spreading agents for formic acid. 9-Tricosene (23 C) is found in the web of some male spiders (Pholcidae) (Schulz S, J Chem Ecol 2013, 39, 1).
Cuticular hydrocarbons have proved to be useful for identifying insect species and differentiating populations. In combination with cuticular hydrocarbons, isoprenoid soldier defensive secretions have been used in some termite species for chemotaxonomic analyses. Thus, analyses have shown that the hydrocarbon profiles of French populations of subterranean termites, Reticulitermes flavipes, were closer to termite populations from Louisiana than to those from Florida (Perdereau E et al., J Chem Ecol 2010, 36, 1189). In ants (Formica exsecta), the chemical profile has been extensively studied across Europe, demonstrating a hydrocarbon profile, composed mainly of homologous series dominated by three n-alkanes (C23, C25, and C27) and three (Z)-9-alkenes (C23:1, C25:1, and C27:1) (Martin SJ et al., J Chem Ecol 2013, 39, 1415). Only the (Z)-9-alkenes have been shown to act as nest-mate recognition cues, with changes in n-alkanes corresponding with task differences. Similar results have been described in other ants and in honeybees. It has been shown that n-alkanes and (Z)-9-alkenes respond to environmental factors, but often in different ways, indicating that their production is controlled by different genetic pathways. Studies of phenotypic variations in various ant population support the primary role of (Z)-9-alkenes as recognition cues and that of n-alkanes, and other cuticular lipids, as anti-desiccants.
Analyses of cuticular lipids of adult Schistocerca shoshone have revealed that insects had the same hydrocarbons, but their relative abundance varied between populations and, within a locality, remained more or less constant over time. It was observed that the cuticular lipids of insects from a population living in an area with high summer temperatures included higher proportions of n-alkanes than those of insects from a less extreme environment (Chapman RF et al., Biochem System Ecol 1995, 23, 383).
Field bioassays enabled the discovery of alkenes as bee sex pheromones and orchid attractants. Thus, (Z)-9, (Z)-11 and (Z)-12 alkenes (C25, C27, C29) elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37). Additive bioassays indicated the importance of the alkene double bond position for controlling pollinator preference.
Several hydrocarbons (octane, nonane, dodecane, hexadecane…) belong to aroma compounds which are found in environmental or food systems (see the website Flavornet).
Several chemical compounds derived from petroleum distillation and refining belong to what is named “mineral oil saturated hydrocarbons”. Among them, some can enter food in many ways (environment, lubricants, food additives, migration from contact materials). They are able to be accumulated in the liver and the lymphoid system, but do not seem to present a public health risk at current levels.
Various hydrocarbons are present in the photosynthetic prokaryotes but in low concentrations. Most species have from 15 to 20 carbon atoms, heptadecane being by far the most predominant in all species. In some species, mono- or di-unsaturated chains (alkenes) were found, in others (Cyanobacteria) methyl-branched alkanes are present.
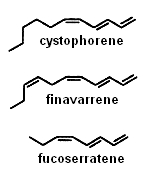
Several alkenes with 8 or 11 carbon atoms and 3 or 4 double bonds play a role in algae gamete attraction (pheromones) : cystophorene in Cystophora sp, finavarrene in Ascophyllum sp and Sphaerotrichia sp, fucoserratene in the brown seaweed Fucus serratus and in the freshwater diatom Asterionella formosa (Bacillariophyceae).
During the investigation of marine aroma components in the brown algae Sargassum thunbergii, characteristic volatile hydrocarbons have been detected. Thus, (6Z,9Z,12Z,15Z,18Z)-1,6,9,12,15,18-henicosahexaene and (6Z,9Z,12Z,15Z-1,6,9,12,15-henicosapentaene have been analyzed (Lu SJ et al., J Oleo Sci 2018, 67, 1463).
Hydrocarbons are also formed as products of fatty acid cleavage during peroxidation processes. Alkanes as well as alkenes appear during hydroperoxide decomposition.
Long-chain (>C25) n-alkyl hydrocarbons are among the most important biomarkers for reconstructing past environmental and climatic conditions. For example, long-chain n-alkanes are considered biomarkers for terrestrial inputs to remote sediments in the South Pacific of the Southern Ocean through long-range aeolian dust transport and in ArabianSea. Nevertheless, it has been demonstrated that long-chain n-alkyl lipids can originate from aquatic microbial sources as they are present in Antarctic lakes, where no vascular plants are present (Chen X et al., Org Geochem 2019, 138:103909).
Chlorinated hydrocarbons are industrial chemical compounds produced in high volume since the 1930s. They are mainly used as plasticisers, flame retardants and lubricants. The main constituents based on n-alkanes, some of them being also based on branched alkanes. Depending on the carbon chain length, mixtures are classified as short-chain (C10–13), medium-chain (C14–17) or long-chain (C18–30) chlorinated hydrocarbons. The chlorine content may be between 30 and 70 % by mass.
Short and medium-chain chlorinated hydrocarbons have a very high persistence, bioaccumulation, very high bioaccumulation and toxicity. It is therefore necessary to assess the exposure of populations to these contaminants. Human exposure to these molecules through mainly food, but dust ingestion, inhalation and skin have already been reported. In France, daily intakes have been estimated to 7.9 and 100 ng per kg of body weight per day for ∑(C10–13) and ∑(C14–17), respectively (Amouro C et al., Food Chem 2025, 488, 144746).
Chlorinated hydrocarbons with more than 15 carbons have been reported in the leaf waxes of halophytic Chenopodiaceae (Grossi V et al., Phytochemistry 2003, 63, 693). Occurrence of long chain chloroalkenes and chloroalkanes with 30 to 36 carbons have been reported in lake sediments from the Galapagos Islands (Zhang Z et al., Org Geochem 2013, 57, 1). The precursor of these long chain lipids remains yet an enigma.
Among hydrocarbons, a variety of forms are described (saturated or unsaturated):
The normal paraffins : their general formula is CH3(CH2)nCH3 (n= 6 – 40 or greater).
Paraffins may have branched chains :
– one methyl group (monobranched), iso-branched hydrocarbons (methyl group on the second carbon) or anteiso-branched hydrocarbons (methyl group on the third carbon)
(as below)
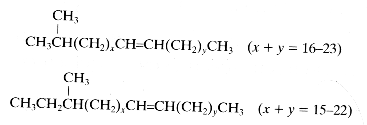
Among the almost 1,000 cuticular hydrocarbons present in ant species, about 200 monomethylakanes and 600 dimethylakanes are used for chemical communication (Martin S et al., J Chem Ecol 2009, 35,1151). Odd chain lengths and positions of methyl at odd carbon numbers are far more numerous than even chain-length compounds. That chemical recognition, fundamental in insect societies, is known for over 100 years in ants and was shown to be based on antennal detection of non-volatile compounds found on cuticle (Fielde AM, PNAS 1901, 53, 521). Since 1987, the nest-mate discrimination systems in several ant species are known to be based on hydrocarbons (Bonavita-Cougourdan et al., J Entomol Sci 1987, 22, 1).
In longhorned beetle, Mallodon dasystomus (Coleoptera, Cerambycidae), cuticular hydrocarbon profiles of females contained 13 compounds that were not present in profiles of males. Among the female-specific compounds, two co-dominant methylbranched alkanes, 2-methylhexacosane and 2- methyloctacosane, are contact pheromes and accounted for 17% of the total hydrocarbons (Spikes AE et al., J Chem Ecol 2010, 36, 943).
– several methyl groups (multibranched), one for each unit deriving from the isoprene formula : CH2=C(CH3)-CH=CH2. Hydrocarbons formed of isoprene units belong to the large group of terpenes.
This chain type is frequently found in several lipid forms, either isolated or combined with other chemical structures. A series of long-chain methylated alkanes (more than 23 carbon atoms), saturated or with one double bond, were identified in settling particles and surface sediments from Japanese lakes and were shown to be produced by planktonic bacteria being thus useful molecular markers (Fukushima K et al., Org Geochem 2005, 36, 311). Laboratory experiments have demonstrated that n-alkanes up to C35 may be formed in the laboratory under hydrothermal conditions (Fischer-Tropsch-type reactions) from formic acid or oxalic acid (Mccollum TM et al., Orig Life Evol Biosph 1999, 29, 153). These results support the theory of the origin of life in hydrothermal systems.
Methoxyalkanes have been identified on bodies or silk of spiders : 1-methoxy-16,20,24,28-tetramethylhentriacontane and 1-methyl-2,24-dimethyloctacosane (Schulz S, J Chem Ecol 2013, 39, 1).
It must be noticed that highly branched and unsaturated (2-5 double bonds) isoprenoids are widespread components in marine sediments (review by Rowland SJ et al., Marine Envir Res 1990, 30, 191; Belt ST et al., Geochim Cosmochim Acta 2000, 64, 3839). The identification of C25 and even of C30 highly branched isoprenoid alkenes in diatoms (Johns L et al., Org Geochem 1999, 30, 1471) have clearly established that they are the source of these compounds found in sediments.
Among the saturated isoprenoids found in geological sediments and oils, the most frequent are pristane (2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-tetramethylhexadecane). Both compounds can be generated diagenetically from the phytol side chain of chlorophyll. Pristane may also derive from the side chain of tocopherols while phytane is also generated by Archaea.
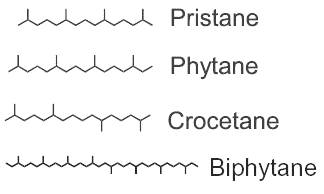
The widespread use of pristane as a biological marker is related to its structural similarity to phytol and its apparent stability, in connection with inability of microorganisms to carry out its anaerobic destruction. pristane is present in photosynthetic organisms, it has been detected in bacteria, algae, and higher plants. Marine sources of pristane include zooplankton, lobster, fish, sharks, sperm whale. Fossil fuels such as coal and petroleum contain this compound. The stable structure persists even in Precambrian rocks and perhaps in extraterrestrial meteorites. The coexistence of microfossils with pristane and phytane in Precambrian rocks is significant to the paleobotanist. Despite this inertness, pristane can be utilized as the sole source of carbon and energy for growth of a coryneform soil isolate ( McKenna EJ et al., PNAS 1971, 68, 1552). It has been suggested that the proportion of the diatom isomeric (E/Z) highly branched isoprenoid may serve as a new proxy for palaeo sea surface temperatures, including in the polar regions (Belt ST et al., Org Geochem 2025, 208, 105056).
Crocetane is formed by four isoprene units arranged symmetrically around a tail-to-tail linkage (2,6,11,15-tetramethylhexadecane). It was initially synthesized and named by Karrer et al. (Helv Chim Acta 1930, 13, 707). Crocetane is mainly present in oils and in methane-rich sediments, as it was shown to be produced by microorganisms utilizing methane as their carbon source. 2,6,10,15,19-Pentamethylicosane differs from crocetane by the addition of a single isoprene unit, joined head to tail, at one end of the molecule. This compound is present in methane-rich sediment and is likely produced by anaerobic methanotrophs.
Phytane and biphytane, present in sediments and petroleum, are thought to derive from ether-lipids (archaeol, caldarchaeol) of Archaea, the only organisms known to possess such structures.
The separation of straight chain and branched chain alkanes is efficient on a micro scale using the urea complex formation as described for fatty acids (Xu S et al., Org Geochem 2005, 36, 1334). That efficient method is based on the urea inclusion directly on the TLC plates and successive elutions with two solvents to separate straight and branched alkanes.
One important member of isoprenoid polyenes is squalene (C30H50) which is a metabolic precursor of sterols and steroids and classified into the triterpenoids. It is also a component of sebaceous lipids (12-15% of sebum weight) found on human skin. It consists of 6 isoprene units and contains 6 trans double bonds. It was discovered in 1906 in shark oil (Tsujimoto M, J Soc Chem Ind Jpn, 1906, 9, 953). It was suggested that squalene and its peroxidized derivatives (6 hydroperoxysqualene are possible) occurring by UV irradiation have an important role in the occurrence of sunburn, and/or protection from sunburn skin damage (Ohsawa K et al., J Toxicol Sci 1984, 9, 151). It has been shown that hydroperoxysqualene induces expression of genes linked to inflammation but its effect may be lowered by gamma-tocotrienol, a form of vitamin E (Nakagawa K et al., Lipids 2010, 45, 833).
Furthermore, it has been suggested that squalene peroxides may play an important part in the pathology of acne, pityriasis versicolor, and skin aging. There is some evidence that squalene reduces colon cancer (Rao et al., Carcinogenesis 1998, 19, 287) and skin cancer (Owen R et al., Food Chem Toxicol 2000, 38, 647). This activity is likely related to its antioxidant effect (Amarowicz R, Eur J Lipid Sci Technol 2009, 111, 411).
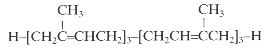
It must be mentioned that if squalene is found in large quantities (from 0.2 to 0.9 g per Kg) in some fish liver oils (shark), it is also found in olive oil (its content may vary from 1 to 40 mg per Kg) where it is used to detect any adulteration. Other vegetable oils contain only traces of this compound (from 0.02 up to 0.3 mg per Kg). Squalene was also found in the epicuticular wax of fruit (grapefruit) and in the hydrocarbon fraction of wheat.
Squalene, and its saturated derivative (squalane) present in skin sebum, are largely used in cosmetics. Squalane is obtained by hydrogenation of the squalene pool isolated mainly from olive oil.
A range of C25 highly branched isoprenoid alkenes were discovered in filtered phytoplankton samples from Arctic waters and from sub-Antarctic waters. These alkenes contained 3–5 double bonds and had structures identified previously from analysis of laboratory diatom cultures.They were also identified in diatoms of the genus Rhizosolenia isolated from seawater collected in the South Atlantic (Belt ST et al., Org Geochem 2017, 110, 65).
It has been shown that presqualene diphosphate, intermediate between farnesyl diphosphate and squalene, carries biological activity in human neutrophiles and serves as a negative intracellular signal preventing superoxide anion generation (Levy BD et al., Nature 1997, 389, 985). An inhibition of phosphatidylinositol 3-kinase in the same cells was also demonstrated (Bonnans C et al., J Exp Med 2006, 17, 203, 857). During that signaling step, squalene diphosphate is transformed into the inactive monophosphate species (Fukunaga K et al., J Biol Chem 2006, 281, 9490).
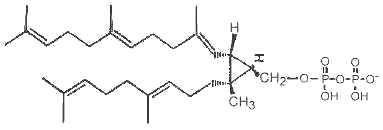 Presqualene diphosphate
Presqualene diphosphate
Two homologous series of trimethylalkanes, the 3,7,11-trimethylalkanes (C34H7), C36H74 and C38H78) and the 4,8,12-trimethylalkanes (C35H72, C37H76 and C39H80) have been described as the major constituents of the cuticular alkanes of the ant, Atta colombica (Martin MM et al., Tetrahedron 1970, 26, 307). Each of these structures combines a reduced polyketomethylene chain and a modified isoprenoid chain, and hence combine structural units from two major metabolic pathways. Hydrocarbons of this type have not been previously isolated from natural sources.
Pheromones with an epoxy ring are biosynthesized from unsaturated hydrocarbons by action of epoxidases specific for one specific double bond. In the case of monoepoxides derived from 3,6,9-trienes, all three kinds of epoxydienes (3,4-epoxy- 6,9-dienes, 6,7-epoxy-3,9-dienes, and 9,10-epoxy-3,6-dienes) have been found in female moths, 9,10-epoxy-1,3,6-triene has been identified as a natural pheromone component from two species in Arctiidae, the fall webworm (Hyphantria cunea) (Tóth M et al., Tetrahedron Lett 1989, 30, 3405) and the mulberry tiger moth (Lemyra imparilis) (Ando T et al., Topics Current Chem 2004, 239, 51). 9,10-Epoxy-(3Z,6Z)-1,3,6-henicosatriene has been identified from a pheromone gland of arctiid species, such as Hyphantria cunea (Yamakawa R et al., J Chem Ecol 2012, 38, 1042).
ALKYNES – In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond or acetylene group. Acetylene compounds or “polyacetylenes” (the latter term is often used) are a general name for a substantial class of natural hydrocarbons, all containing one or more carbon−carbon triple bond functionalities in their molecules. Naturally occurring polyacetylenes feature a wide range of structural diversity and are widespread in plants and animals (Minto RE. et al., Prog. Lipid Res 2008, 47, 233). In the marine environment, they have been isolated mainly from algae and invertebrates, and have displayed a broad array of biological properties, including antifungal activity, antimicrobial activity, HIV reverse transcriptase inhibition, and cytotoxicity. In addition, important ecological roles, such as inducing metamorphosis of ascidians’ larvae, preventing fouling by barnacle larvae, and inhibiting fertilization of starfish gametes, have been ascribed to these metabolites. Polyacetylenes of marine origin, including their chemistry and bioactivity, were reviewed in an important report (Zhou ZF et al., Chem Rev 2015, 115, 1543).
Acetylenes isolated from algae are mostly C-15 metabolites, a group of compounds of great importance. Almost all of them were isolated from the red algae belonging to the Laurencia genus, making them characteristic metabolites of the Laurencia algae. As an example, laurencenyne has been isolated from Laurencia okamurai (Kigoshi H et al.. Tetrahedron Lett 1981, 22, 4729).
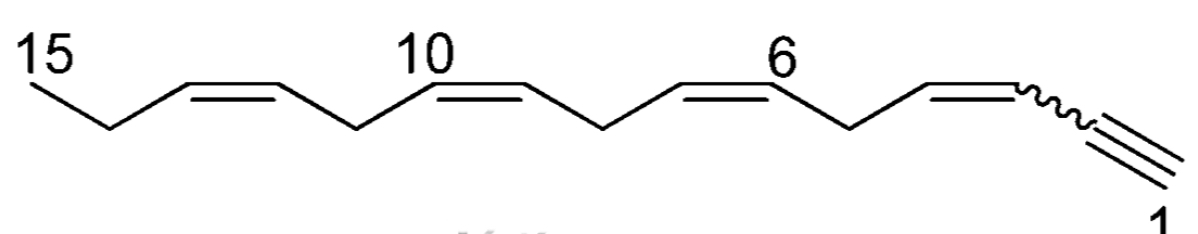
Laurencenyne
Asymmetric poly-acetylenes with long chains ranging from n = 16 to n = 48 are ubiquitous in marine sponges, and they are believed to play a crucial role in the sponge metabolism.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire