
The Nobel Nobel prize for chemistry for 1928 was awarded to Adolf Windaus “for his studies on the constitution of the sterols and their connection with vitamins“. The vitamin in question was vitamin D and Windaus was the first scientist to receive an award mentioning vitamins.
A comprehensive review of the vitamin D history viewed through the contribution of Windaus may be found in a paper by Wolf G (J Nutr 2004, 134, 1299).
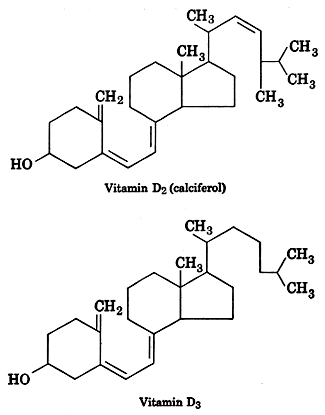
Vitamin D2 (calciferol) originates from irradiation at about 260nm of ergosterol while vitamin D3 is formed by irradiation of a provitamin molecule (7-dehydrocholesterol) present in the skin and gut lining. From a physiological point of view, Steenbock H et al. discovered the formation of an anti-rachitic factor in rats under UV irradiation (J Biol Chem 1924, 61, 405). Vitamin D3 was isolated around 1936 (Brockmann) from the very potent liver oil of the tuna fish. The unsaponifiable fraction was partitioned between 90% methanol and petroleum ether to separate vitamins A and D. The residue from the petroleum phase contained vitamin D3 to be purified by multiple chromatography. The total synthesis was done around 1952 (Woodward, J Amer Chem Soc, 74, 4223). When tested on rats, the two vitamins are equally potent (vitamin D3 is more active than D2 in the chick test). The international unit has been so defined as 40 i.u./µg. Natural fish liver oils vary from about 100 i.u./g in cod to 200,000 i.u./g in tuna fish.
The two commonly available forms of vitamin D supplement for maintaining vitamin D status or treating vitamin D deficiency or insufficiency are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). In Spain, calcifediol has been used for these same purposes for more than 40 years and recently became available in several other European countries (e.g., Poland, The Netherlands, Belgium) and in Latin America.
DeLuca HF (Holick M F et al., Biochemistry 1971, 10, 2799), Norman AW (Biochem Biophys Res Commun 1971, 42, 1082), and Kodicek E (Fraser DR et al., Nature 1970, Nature 228, 764) independently reported the existence of an active metabolite, 1,25-dihydroxyvitamin D3, which was produced in the kidneys. From these observations it was surmised that vitamin D3 was hydroxylated in the liver to become 25-hydroxyvitamin D3 (calcifediol). That immediate precursor metabolite of its active metabolite (calcitriol) has also been shown to bind to the vitamin D receptor with weaker affinity than calcitriol, thus exerting gene-regulatory properties (Donati S et al., Nutrients 2023, 15, 4409). Calcifediol is the major circulating form of the vitamin, and it is then converted to 1,25-dihydroxyvitamin D3 (calcitriol) in the kidneys. Thus, this final metabolic product is now considered as the metabolically active form of vitamin D3, which carried out its functions in initiating intestinal calcium transport. As the generation of vitamin D3 by UV-mediated photosynthesis is the main source of this secosteroid compound, it can be considered that vitamin D3 is not a true vitamin but a true hormone.
People with mutations in the enzyme which converts 7-dehydrocholesterol into cholesterol (a reductase), show elevated levels of 7-dehydrocholesterol. These people suffer from the Smith–Lemli–Opitz syndrome which can induce physical, cognitive and behavioural symptoms of varying severity (Wikipedia). Prenatally, the syndrome may be diagnosed upon finding an elevated 7-dehydrocholesterol/total sterol ratio in fetal tissues, or increased levels of 7-dehydrocholesterol in amniotic fluid.
It has been shown that vitamin D3 is present in plants, especially in species belonging to the family Solanaceae. The first demonstration that Solanum glaucophyllum living in South America is a source of 1,25-dihydroxyvitamin D3 has been done in 1977 (Napoli JL et al., J Biol Chem 1977, 252, 2580). The concentration was estimated to 230 pg/g of tissue. Later, it was demonstrate that grass potentially can be a significant source of vitamin D2 (average content was 2ng/g fresh weight) for grazing animals and animals fed on silage and hay (Japelt RB et al., J Agric Food Chem 2011, 59,10907). Vitamin D3 and its hydroxylated metabolites were isolated from leaves of Solanum glaucophyllum but also of tomato (S. lycopersicum) and bell pepper (Capsicum annuum) (Japelt RB et al., Food Chemistry 2013, 138, 1206). Vitamin D3 and 25-hydroxy vitamin D3 were found in the leaves of all plants after UVB-treatment. S. glaucophyllum had the highest content, 200 ng vitamin D3/g dry weight and 31 ng 25-hydroxy vitamin D3/g dry weight, and was the only plant that also contained 1,25 dihydroxy vitamin D3 in both free (32 ng/g dry weight) and glycosylated form (17 ng/g dry weight).
The foods that are the richest in vitamin D are fatty fish (5-20 microg/100 g) and eggs, with smaler amounts being present in milk, butter, and some meats. The concentrations in several foods may be found on the French CIQUAL table and on the Australian and New Zealand online searchable NUTTAB data base.
It is now well known that vitamin D is a central player in calcium and bone metabolism, but more recently, important immunomodulatory effects have been attributed to this vitamin (Baeke F et al., Mol Aspects Med 2008, 29, 376). It plays also a key role in the regulation of calcium and phosphate homeostasis, and its metabolism involves numerous complex pathways (Nardin M et al., Biomedicines 2024, 12, 768). Furthermore, vitamin D deficiency results in hyperparathyroidism, which increases bone resorption, reducing the bone mineral content, and increases the risk of fracture.
Vitamin D deficiency/insufficiency has been associated with muscle weakness and a high incidence of various chronic diseases such as cardiovascular disease, cancer, multiple sclerosis, and type 1 and 2 diabetes (Zittermann A et al., Nutrients 2010, 2, 408). Most importantly, low vitamin D status has been found to be an independent predictor of all-cause mortality (Zittermann A et al., Curr Opin Clin Nutr Metab Care 2009, 12, 634). A review of the new trends in the physiological actions of vitamin D has been released in 2009 (Dixon KM et al., Int J Biochem Cell Biol 2009, 41, 982) and may be found in my book (Lipids – Nutrition and health, Claude Leray, CRC Press). The role of vitamin D in cardiovascular and bone health has been largely summarized in terms of its effects on certain systems, but with some debate.
The associations of both serum vitamin D status and supplementation with all-cause dementia, Alzheimer’s disease, and vascular dementia incidence has been reviewed in a study made on 269,229 participants, aged 55 to 69, from the UK Biobank (Chen LJ et al., Am J Clin Nutr 2024, 119, 1052). Findings indicate potential benefits of vitamin D supplementation for dementia prevention. The potential use of vitamin D3 for its anti-ageing effects has been reviewed (Santa K et al., Int J Mol Sci 2024, 25, 2125).
It has been observed that a 4-years of supplementation with 2000 IU/day vitamin D3 reduced telomere attrition, suggesting that it might have a role in counteracting telomere erosion or cell senescence (Zhu H et al., Am J Clin Nutr 2025, online 21 May).
It has been observed that vitamin D intake was associated with older persons frailty (Marcos-Pérez D et al., Nutrients 2020, 12, 2286). It was also shown that improvements in dietary inflammation and increased vitamin D levels may help alleviate frailty (Wang Y et al., PLoS One 2025, 20, e0327251). Thus, people should pay more attention to the diet pattern of older adults.
Vitamin D3 was developed as a rodenticide in the 1970s. It has a relatively low risk of secondary poisoning and low toxicity to birds and other mammals. 25-Hydroxycholecalciferol is the most biologically active form of vitamin D3 which can cause calcification of blood vessels and death of the rat from heart failure. Low doses of cholecalciferol have been added to bait-containing anticoagulants to increase their effectiveness.
Cholecalciferol was identified as an endocrine disruptor during its assessment by the European Chemicals Agency under the Biocides Regulation (EU) No 528/2012. However, ANSES stresses that the doses of cholecalciferol used in biocidal products to eradicate rodents are far higher than the doses of vitamin D provided by a normal diet, including foods fortified with vitamin D. The Agency believes that identifying cholecalciferol as an endocrine disruptor on food product labelling would contribute to an incorrect perception of the risk and could deter some people from consuming foods containing vitamin D. Such labelling could therefore make it harder to meet adequate nutritional requirements for vitamin D. According to the INCA 3 study, the average dietary intake of vitamin D in the French adult population is 3.1 μg/day for adults aged 18-79 years, well below the adequate intake of 15 μg/day.
Calcitriol has antimicrobial activity against Helicobacter pylori, and 100 μM calcitriol kills this bacterium within 2 h. In addition, clinical results showed that serum 25-hydroxyvitamin D levels lower than 20 ng/mL might affect the ability of quadruple therapy to kill H. pylori, and higher levels were associated with effective killing (Zhou A et al., Front Cell Infect Microbiol 2020, 10, 566730). The molecular mechanism of that action has been more recently examined (Cao Z et al., Biochem Biophys Acta – Mol cell bill Lipids 2024, 1869, 159430).
Initially, the determination of vitamin D was based on a reaction between antimony trichloride giving a yellow color with l max 500nm. Removal of vitamin A and E was necessary. Partition chromatography is actually used with success.
|
vitamin D2 |
Vitamin D3 |
|
| Molecular weight |
396.6 |
384.6 |
| l max |
265 |
265 |
| Molar extinction coefficient |
18,200 |
18,200 |
| m.p. |
116°C |
83°C |
For humans, the requirements are about 100 i.u./day for a man but 400 i.u./day for a child or a pregnant woman.
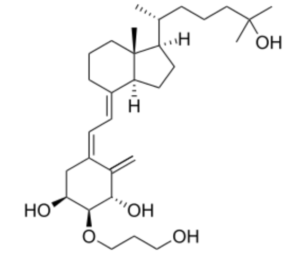
 Calcitriol
Calcitriol
The analysis of the vitamin D metabolites is dominated by immunoassays and receptor binding assays but an efficient profiling of major vitamin D metabolites has been described using a Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry (Aronov PA et al., Anal Bioanal Chem 2008, 391, 1917). A review of that approach has been reviewed (Higashi T et al., J Chromatogr B 2010,878, 1654).
A chemically vitamin D3 derivative, eldecalcitol (trade name: Edirol), has been proposed and aproved in Japan to reduce calcium reabsorption into the body from bones (osteoporosis), therefore increasing bone mineral density, and to increase calcium absorption in intestines (Noguchi Y et al., Clin Interv Aging 2013,. 8, 1313). This analog of calcitriol contains a hydroxypropyl group in the lower cyclohexane ring (Wikipedia). It has been determined that its binding to the vitamin D binding protein is 2.7-fold more potently than calcitriol.
 Eldecalcitol
Eldecalcitol
If vitamin D2 and D3 are the two main forms of vitamin D, they are not the only ones. By UVB action, other tetracyclic steroids are converted into secosteroids which are structurally similar to cholecalciferol. The best known of these are vitamin D4 originating from 22,23-dehydrocholesterol and D5 (sitocalciferol) originating from 7-dehydrositosterol (Wikipedia). The interest in vitamin D5 comes from its anti-neoplastic capacity (from its hydroxylated metabolite in the 1 alpha position, 1 alpha-OH vitamin D5) (Mehta RG et al., Eur J Cancer 2004;40, 2331). In vivo and in vitro studies have shown its inhibitory effect against mammalian carcinogenesis in mice.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire