
AMINO COMPOUND-CONTAINING LIPIDS
Aminoalcohol-containing lipids
A – AMINO ACID-CONTAINING LIPIDS (Simple Lipoamino acids and lipopeptides)
Some bacteria species are known to contain in their inner and outer membranes amphipatic lipids based on one or two amino acids linked to a fatty acid through an amide bond and sometimes another through an ester bond. Thus, they have a structural similarity to ceramides or lipopolysaccharides.
Some of these conjugates of lipids and amino acids were also found in plants and in mammalian tissues.
New forms of these compounds are frequently described in marine organisms. Among them, unusual structures formed of fatty acids and amino acid derivatives (bromotyrosine) are isolated from sponges, they were named mololipids.
Surface-active molecules containing several amino acids and one or two fatty acids are produced by bacteria, but also in mammals, and are known as lipopeptides.
Several types of lipoamino acids have been identified :
1 – Lipids containing serine
The best known is serratamic acid or hydroxydecanoyl serine.
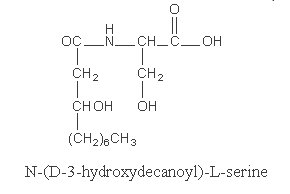
This compound was detected in Serratia species of bacteria (Cartwright NJ, Biochem J 1957, 67, 663).
It was suggested that this compound may contribute to the virulence of the bacteria (inhibition of phagocytosis, hemolytic activity). A hydroxydodecanoyl derivative is also known.
Another form was described in an opportunistic pathogen Flavobacterium (Kawai Y et al., Eur J Biochem 1988, 171, 73). In this compound (named "flavolipin"), serine is amide-linked to 3-hydroxyisoheptadecanoic acid which is esterified to isopentadecanoic acid. It has high hemaglutinating activity.
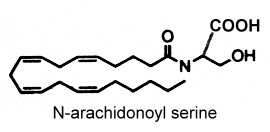
N-arachidonoyl serine was isolated from bovine brain (Milman G et al., PNAS 2006, 103, 2428). This compound binds very weakly to cannabinoid and vanilloid receptors but it was identified as an endocannabinoid-like constituent with vasodilatory properties.
A N-acyl homoserine lactone (with an oxohexanoyl acyl group) was first described in Photobacterium fischeri and identified as an autoinducer of luciferase synthesis when excreted into the medium (Eberhard A et al., Biochemistry 1981, 20, 2444). Since these early observations, it has become evident that this lipo-amino acid is only one member of a growing family of acyl homoserine lactones which are produced by a wide spectrum of bacteria and are responsible for the regulation of diverse physiological processes in the corresponding producer organisms.
That family differs in either the presence or absence of an acyl chain C-3 substituent (oxo or hydroxy) or the length of the N-acyl side chain. One of the best studied among these molecules is N-(3-oxododecanoyl) homoserine lactone produced by Pseudomonas aeruginosa (Pearson JP et al., Proc Natl Acad Sci USA 1994, 91, 197) :
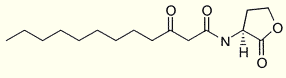
This molecule was shown to activate several virulence genes and to be a component of the "quorum-sensing" system involved in nearly all gram-negative bacteria for controlling gene expression in response to population density. The term "quorum sensing" was coined by WC Fuqua in 1994 to describe the environmental sensing system that allows bacteria to monitor their own population density (WC Fuqua et al., J Bacteriol 1994, 176, 269). Later, quorum signaling molecules were shown to be implicated in the regulation of an array of important bacterial phenotypes including cell division, production of virulence factors, motility, sporulation, nodulation, plasmid transfer, antibiotic production, bioluminescence, and biofilm formation. It was also shown that some could be an important immune modulator (Telford G et al., Infect Immun 1998, 66, 36 and Chhabra SR et al., J Med Chem 2003, 46, 97). It has been demonstrated that N-(3-oxododecanoyl) homoserine lactone can promote the upreglation of proinflammatory cytokines and chemokines through the inhibition of PPARg receptors (Jahoor A et al., J Bacteriol 2008, 190, 4408). These results suggest that PPARg agonists could e employed as anti-inflammatory therapeutics for Pseudomonas aeruginosa infections.
On another hand, there is now a considerable interest in the ability of specific enzymes to inactivate these molecules, thus decreasing the virulence of a number of pathogenic bacteria (Roche DM et al., Microbiology 2004, 150, 2023). Bacterial quorum sensing molecules are also recognized by eukaryotes. In this case, the cross-kingdom interaction can lead to specific adjustment and physiological adaptations in the colonized eukaryote (Hartmann A et al., J Chem Ecol 2012, 38, 704).
It was shown that acylhomoserine lactones could be produced by bacteria associated with cultivated mushrooms (Prashanth SN et al., J Agric Food Chem 2011, 59, 11461).
The role played by acylhomeserine lactones in the rhizosphere signaling has been reviewed (Venturi V et al., Trends Plant Sci 2016, 21, 187).
Another type of homoserine lactone but with a coumaroyl group instead of an acyl group has been described in a photosynthetic bacterium Rhodopseudomonas palustris (Schaefer AL et al., Nature 2008, 454, 595). That compound (p-coumaroyl-homoserine lactone), synthesized from environmental p-coumaric acid, is used by the bacteria as quorum-sensing signal, which function with a receptor to control the expression of specific genes. It was also synthesized by other bacteria such as Bradyrhizobium and Silicibacter.
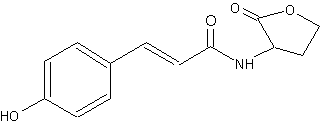
p-Coumaroyl-homoserine lactone
2 – Lipids containing ornithine
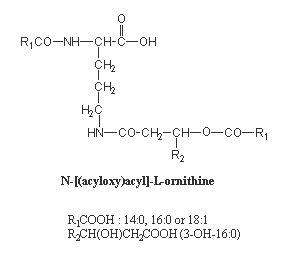
The most common structure of lipoamino acids shown below has a 3-hydroxy amide-linked acid which is itself esterified by a second fatty acid, generating an acyloxyacyl function. That structure is reminiscent of that found in lipid A of Gram-negative bacteria. They are found among others in several species of Bordetella, Pseudomonas, Flavobacterium and Achromobacter.
This ornithine-containing lipid was recently found to be a strong stimulant for macrophages and, taking in account its biological activities, is expected to be used as a potent and nontoxic adjuvant in immunology studies (Kawai Y et al., FEMS Immunol Med Microbiol 1999, 23, 67). Similar molecules have been shown to protect animals from lethal endotoxemia (Kawai Y et al., Infect Immun 1991, 59, 2560).
Other forms were found in various Flavobacterium, opportunistic pathogens which cause infantile meningits (Kawai Y et al., Eur J Biochem 1988, 171, 73-80). In these compounds, ornithine is amide-linked to a hydroxylated fatty acid (3-hydroxyisoheptadecanoic acid) which is itself esterified to isopentadecanoic acid or 2-hydroxyisopentadecanoic acid. They were shown to exhibit high hemagglutinating activity.
Another form of ornithine-containing lipid is found in photosynthetic bacteria and contains amide and ester linkages between the amino acid and the fatty acids (one being normal, the other being hydrolylated).
One form synthesized by several bacteria species (Bordetella, Pseudomonas, Achromobavter) was found to be a strong stimulant for macrophages (Kawai Y et al., FEMS Immunol Med Microbiol 1999, 23, 67).
More complex forms containing ornithine linked to a fatty acid and a long-chain fatty alcohol were also described.
An interesting feature of these molecules appears to be in the membranes functionally interchangeable with phosphatidylethanolamine. Thus, in a culture of Pseudomonas, phosphorus deficiency increases the acylornithine production to the exclusion of phospholipids (Minnikin DE et al., FEBS Lett 1974, 43, 257).
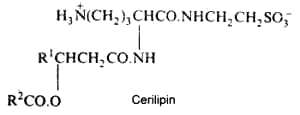
The bacterial Cerilipin described in Gluconobacter cerinus has an ornithine-containing lipid core but with an additional amide-linked taurine (Tahara Y. et al., Agric Biol Chem 1976, 40, 243). It was shown to exhibit excellent biosurfactant activity.
3 – Lipids containing tyrosine
A new series of lipophilic bromotyrosine derivatives was isolated as a white waxy extract from a Verongid sponge from Hawaii. Named mololipids, the extract contained a group of molecules consisting of bis-amides between a bromotyrosine derivative (formerly known as moloka’iamine) and long chain fatty acids ranging in size from C14 to C20 (Ross SA et al. J Nat Prod 2000, 63, 501). These compounds were shown to display anti-HIV activity.
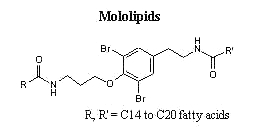
Pure samples of mololipids with defined fatty acid composition have been prepared by total synthesis for pharmacological investigations (Schoenfeld RC et al., Bioorg Med Chem Lett 2000, 10, 2679).
A glycine-containing lipoamino acid was described for the first time in a gliding bacteria, Cytophaga johnsonae.
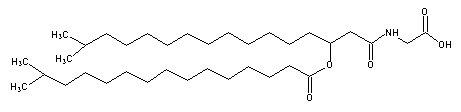
Its structure showed an iso-3-hydroxy heptadecanoic acid, amide linked to glycine and esterified to isopentadecanoic acid, it formed about 6% of the total bacteria lipids (Kawazoe R et al., J Bacteriol 1991, 173, 5470).
Another glycine-containing lipoamino acid, a conjugate of arachidonic acid and glycine (N-arachidonoylglycine), was shown to be active against pain (Burstein SH et al., Prostaglandins Other Lipid Mediat 1999, 61, 29). It was later shown to be present in bovine and rat brain as well as in other tissues, Its biosynthesis and degradation being further observed to occur in the rat brain (Huang SM et al., J Biol Chem 2001, 276, 42639). Consistent with its high levels in skin and neural tissues, N-arachidonoylglycine is capable of suppressing pain via a peripheral action, suggesting that it may serve endogenously to regulate pain.
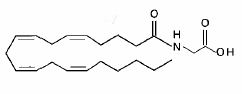
This lipoamino acid was first synthesized (Sheskin T et al., J Med Chem 1997, 40, 659) as a structural analog of the endogenous cannabinoid anandamide and later was shown to cause hot plate analgesia in mice (Burstein SH et al., Prost Lipid Mediat 1999, 61, 29). Thus, one possible physiological function of N-arachidonoylglycine is pain suppression (Huang SM et al., J Biol Chem 2001, 276, 42639).
It was reported that cyclooxygenase-2 selectively metabolized N-arachidonoylglycine to PGH(2) glycine and hydroxyeicosatetraenoic glycine (Prusakiewicz JJ et al., Biochem Biophys Res Com 2002, 296, 612). These results suggest a possible role for cyclooxygenase-2 in the regulation of N-arachidonoylglycine levels and the formation of a novel class of eicosanoids.
Two other arachidonyl amino acids were shown to be natural constituents in mammalian brain: N-arachidonyl g-aminobutyric acid and N-arachidonoylalanine (Huang SM et al., J Biol Chem 2001, 276, 42639).
Lipstatin, a new and very potent inhibitor of pancreatic lipase (the key enzyme of intestinal fat digestion) was isolated from Streptomyces toxytricini (Weibel EK et al., J Antibiot 1987, 1081). Lipstatin contains a beta-lactone ring, probably accounting for the irreversible lipase inhibition, which carries two aliphatic residues with chain lengths of 6 and 13 carbon atoms. One of the side chains contains two isolated double bonds and a hydroxy group esterified to N-formyl-leucine.
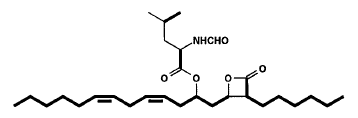
Several research were focused on the biosynthesis of that potent molecule (Eisenreich W et al., J Biol Chem 1997, 272, 867 and J Med Chem 2003, 46, 4209). Data indicate that the lipstatin molecule can be assembled by Claisen condensation of octanoyl-CoA with 3-hydroxy-tetradecanoyl-CoA formed from linoleic acid.
The tetrahydro derivative of lipstatin, Orlistat (Xenical), is used for the treatment of severe obesity in forming a covalent adduct with a serine moiety of human pancreatic lipase by transesterification.
In search of surfactants, it was shown that among several lipoaminoacids synthesized by coupling stearic acid with the a-amino group of an amino acid, N-stearoyl proline had the most efficient surface-active properties and is highly biodegradable. It has antimicrobial activity against several Gram-positive (Staphylococcus aureus, Micrococcus luteus, and Bacillus cerceus), and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria, and against the yeast Candida albicans. Thus, it has a potential utility as biostatic additive in commercial products (Sivasamy A et al., JAOCS 2001, 78, 897).
7 – Lipids containing glutamine
Volicitin, N-(17-hydroxylinolenoyl)-1-glutamine, has been identified in the oral secretions of caterpillars (Spodoptera exigua) shewing on corn (Alborn HT et al., Science 1997, 276, 945). That compound causes the plant to emit other volatile compounds which attract parasitoids or predators (wasps) of caterpillars. It has been determined that the plant provides linolenic acid which is conjugated onto glutamine molecule by the caterpillar (Pare PW et al., PNAS 1998, 95, 13971). Volicitin exhibited the widest range of phytohormone and volatile inducing activity, which spanned maize (Zea mays), soybean (Glycine max), and eggplant (Solanum melongena)
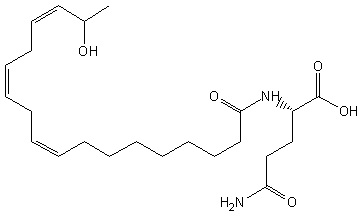
Volicitin
A new structural class of fatty acid amides, N-acyl taurines, has been described in the central nervous system of mice (Saghatelian A et al., Biochemistry 2006, 45, 9007).
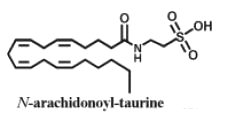
The fatty acids have a carbon chain of up to 20 carbon atoms. In peripheral tissues, N-acyl taurines are also present but have a polyunsaturated acyl chain (e.g., C20:4, C22:6). Their concentration rose more than 10-fold following inactivation of the catabolic enzyme fatty acid amide hydrolase. That dramatic elevation in endogenous levels of N-acyl taurines suggests the existence of a second major lipid signaling system regulated by the fatty acid amide hydrolase in vivo. N-acyl taurines were found to activate multiple members of the transient receptor potential (TRP) family of calcium channels in kidney, including TRPV1 and TRPV4.
It was shown that N-acyl taurines may be a substrate for cyclooxygenases and lipoxygenases resulting in oxidative metabolites of N-arachidonoyl taurine which may have their own biological function (Turman MV et al., Biochemistry 2008, 47, 3917). Metabolism of arachidonoyl taurine by murine macrophages was analyzed, that compound being converted primarily to 12-HETE-taurine. Oxidative metabolism of polyunsaturated N-acyltaurines may thus represent a pathway for the generation or termination of novel signaling molecules.
N-Arachidonoyl taurine and N-oleoyl taurine have been shown to induce a significant reduction in proliferation of PC-3 cells, a human prostate adenocarcinoma cell line (Chatzakos V et al., Lipids 2012, 47, 355).
9 – Lipopeptides
A large number of cyclic lipopeptides including decapeptide antibiotics (gramicidins) and lipopeptide antibiotics (polymyxins), produced by Bacillus brevis (Marahiel M et al., Eur J Biochem 1977, 99, 49), respectively, possess remarkable surface-active properties.
As an example, the cyclic lipopeptide surfactin, produced by B. subtilis, is one of the most powerful biosurfactant known today. It lowers the surface tension from 72 to 27.9 mN/m at concentrations as low as 0.005% (Arima K et al., Biochem Biophys Res Commun 1968, 31, 488). Surfactin is composed of a seven-amino-acid ring structure coupled to one molecule of 3-hydroxy-13-methyl-tetradecanoic acid.

A similar biosurfactant is produced by Bacillus licheniformis, with a lipophilic fatty acid moiety (4 different chain lengths) joined via a lactone linkage to a hydrophilic peptide ring structure (Jenny K et al., J Appl Microbiol Biotechnol 1991, 36, 5).

Cyclic lipopeptides are generally composed of seven amino acid units and a 3-hydroxy or 3-amino fatty acid. They have received considerable attention as key compounds in biocontrol due to their strong antifungal or antimicrobial activity, low toxicity, and high biodegradability compared to chemical pesticides. They are mainly described in Bacillus species where several groups are found : the iturin group including iturin, mycosubtilisin, bacillomycin, the octapeptide group and the polymyxin group (polymyxins).
Polymixins has a peptide ring attached to a peptide chain ending with a branched fatty acid (6-methyloctanoic acid in polymyxin B). Polymyxin B binds to the lipid A portion of lipopolysaccharide and also to phospholipids, thus it is an antibiotic used to treat urinary, meninges and bloodstream infections caused by Gram-negative bacteria (the cell membrane is not exposed in this group).
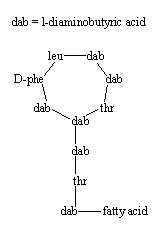
Polymyxins
Biosurfactants in these groups have been noted for their bactericidal or fungicidal activity, their surfactant properties being of only minor interest.
Three cyclic lipopeptides (n = 12, 13 or 14) were isolated from Bacillus amyloliquefaciens (Romano A et al., J Nat Prod 2011, 74, 145). These compounds represented about 60% of the total cell lipids. They are heptapeptides, N-acylated to the N-terminal by an (R)-3-hydroxy fatty acid with linear alkyl chains from 16:0 to 18:0. One of them (n = 14) displayed strong and dose-dependent antifungal activity against the plant pathogenic fungus Fusarium oxysporum.
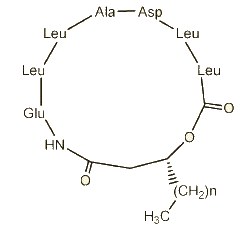
Lipopeptide from Bacillus amyloliquefaciens
A lipopeptide antibiotic, tauramamide, was isolated and characterized from culture of Brevibacillus laterosporus (Desjardine K et al., J Nat Prod 2007, 70, 1850). Its structure was described to be:
7-methyloctanoyl-Tyr-Ser-Leu-Trp-Arg
This lipopeptide has shown potent and selective inhibition of pathogenic species.
Several cyclic tripeptides have been isolated from the halotolerant fungus Aspergillus sclerotiorum (Zheng J et al., J Nat Prod 2010, 73, 1133). Some of them showed selective antifungal activity against Candida albicans.
These molecules are formed in the cytoplasm by transfer of the acyl moiety of acyl-CoA to carnitine (carnitine palmitoyltransferase in the mitochondrial membrane). Acyl carnitines are then transferred into mitochondria and hydrolyzed to release acyl-CoA into the matrix, where they are subject to beta oxidation.
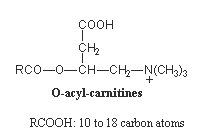
It has been determined that patients with peroxisomal biogenenesis disorders have a characteristic profile of blood acyl carnitines with increases in very long-chain dicarboxylylcarnitines (16 and 18 carbon atoms) and very long-chain monocarboxylylcarnitines (24 and 26 carbon atoms) (Rizzo C et al., Pediatr Res 2003, 53, 1013). Similar observations were recorded in urine of patients affected by these disorders (Duranti G et al., Clin Chim Acta 2008, 398, 86).
C – DOPAMINE-CONTAINING LIPIDS
If the existence of acylated dopamine was previously hypothesized, N-arachidonoyldopamine was the first compound in this group of lipid mediators to be determined in mammalian nervous tissue (Huang SM et al., PNAS 2002, 99, 8400).
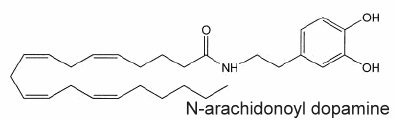
This lipid appears similar to capsaicin (the pungent ingredient of "hot" chili peppers) not only with respect to its chemical structure (a vanillylamine moiety linked to an unsaturated acyl chain via an amide bond) but also to its potency at vanilloid receptors of type 1 present in peripheral nerves and in several brain areas (striatum, hippocampus, cerebellum).
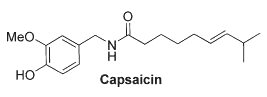
N-Arachidonoyldopamine was also shown to activate cannabinoid CB1 receptors (Bisogno T et al., Biochem J 2000, 351, 817). Furthermore, it has been shown that this lipid has a potent anti-HIV activity (Sancho R et al., J Immunol 2005, 175, 3990).
Further analysis of bovine brain indicated the existence of another potent capsaicin-like lipid, N-oleoyldopamine, which is similar to N-arachidonoyldopamine in its chemical structure and activity on vanilloid receptors (Chu CJ et al., J Biol Chem 2003, 278, 13633).
D – SEROTONIN CONTAINING LIPIDS
Carboxylic acid-5-hydroxytryptamides were described as the main constituents of the waxy layer at the surface of green coffee beans (0.5-2.4 g/kg). They are formed by amides of serotonin (5-hydroxytryptamine, 5-HT) and fatty acids with different chain lengths.
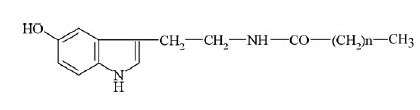
The first amides to be described had fatty acids with 20, 22, and 24 carbon atoms (Harms U et al., Z Lebensm Unters Forsch 1968, 138, 75). Later, several other species were described with a fatty acid from C14 to C22. Technological treatments were initiated to removed that waxy layer, thus preventing potential irritating effects on certain coffee drinkers.
The presence of oleoyl serotonin, palmitoyl serotonin, stearoyl serotonin, and arachidonoyl serotonin was described in the digestive tract (jejunum and ileum) of pig and mouse (Verhoeckx K et al., Biochim Biophys Acta 2011, 1811, 578). Their formation in vitro was stimulated by the addition of serotonin and the pattern of formation was dependent on the relative amount of fatty acids in the diet. Data showed that several of the serotonin conjugates are able to inhibit glucagon-like peptide-1 secretion and fatty acid amide hydrolase activity. It was suggested that N-acyl serotonins are a novel class of lipid mediators regulating intestinal function.
E – AMINOALCOHOL-CONTAINING LIPIDS
The aminoalcohol may be linked to the fatty acid through :
– an amide bond
or
– an ester bond
1 – Aminoalcohol linked as an amide to the fatty acid
N-Acylethanolamines (NAEs) were first reported as constituents of soy lecithin and peanut meal and as a anti-inflammatory agent (Kuehl FF et al., J Am Chem Soc 1957, 79, 5577).
Long-chain N-acylethanolamines are ubiquitous trace constituents of animal and human cells, tissues and body fluids, Their cellular levels appear to be tightly regulated and they accumulate as the result of injury (Berdyshev EV et al., Biochem J 2000, 346, 369). They are found at only very low concentrations. Thus, in rat plasma, the concentrations of palmityl- and oleylethanolamide and arachidonoyl were found to be 17, 8 and 5 pmol/ml, respectively. Somewhat higher concentrations are reported in brain and other tissues. Similar lipids have also been found in fish, molluscs, slime moulds, and certain bacteria.
Saturated and monoenoic N-acylethanolamines may also function as intracellular messengers by activating specific kinases and interacting with the signaling pathways mediated by ceramides. As an example, oleoylethanolamide may be a lipolysis stimulator via activation of the nuclear peroxisome proliferator-activated receptor, PPAR-a (Fu J et al., Nature 2003, 425, 90) and an endogenous regulator of food intake, and could have some potential as an anti-obesity drug (Guzman M et al., J Biol Chem 2004,279, 27849). Preclinical studies have shown that palmitoylethanolamide can produce antidepressant and anxiolytic effects in animal models (Crupi R et al., CNS Neurol Disord Drug Targets 2013, 12, 989). This compound could have potential antidepressant effects in the treatment of human depression (Coppola M et al., Med Hypotheses 2014, 82, 507).
A review of biological functions and metabolism of oleoylethanolamide may be consulted (Thabuis C et al., Lipids 2008, 43, 887).
N-arachidonoyl ethanolamine (named also anandamide) is the first endogenous ligand to be reported at the end of 1992 (Devane WA et al., Science 1992, 258, 1946). It acts as a partial CB1 agonist and only as a weak CB2 agonist. and is formed in the brain from a molecule of N-acyl phosphatidylethanolamine (Bisogno T et al., Pharmacol Biochem Behav 2005, 81, 224). Anandamide is now considered as the main ligand of the cannabinoid receptor (see the ethanolamine glycerolipid chapter)
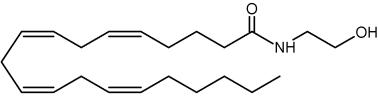
Anandamine
Additional endogenous lipids discovered that show affinity for cannabinoid receptors include dihomo-g-linolenoyl ethanolamide and docosatetraenoyl ethanolamide (Hanus L et al., J Med Chem 1993, 36, 3032).
It has been shown that C18-N-acylethanolamine (based on 18:1, 18:2 and 18:3) are present in ganglia and vascular tissue and, while poor ligands at cannabinoid receptors, they activate the vanilloid 1 receptor (Movahed P et al., J Biol Chem 2005, 280, 38496).
It was shown that 3T3-L1 adipocytes are able to convert docosahexaenoic acid and eicosapentaenoic acid to their N-acyl ethanolamine derivatives (Balvers MGJ et al., Biochim Biophys Acta 2010, 1801, 1107). Docosahexaenoyl ethanolamine was detected in human plasma.
N-Eicosapentaenoyl ethanolamide has been shown in Caenorhabditis elegans to be a signal that coordinates nutrient status with metabolic changes that ultimately determine lifespan (Lucanic M et al., Nature 2011, 473, 226)..
N-Acylethanolamines are also minor but ubiquitous components of plant tissues, but they are especially abundant in some desiccated seeds (Venables BJ et al., Phytochemistry 2005, 66, 1913). The fatty acids have up to three double bonds and 12 to 18 carbon atoms, linoleic acid being the predominant species (Chapman KD et al., Plant Physiol 1998, 116, 1163). Their total contents in seeds range from about 0.2 to 40 mg/g fresh weight with no relationship to phylogeny, their concentration being depleted during germination and seedling development. Some species (Medicago truncatula, Glycine max) could be good candidates for natural sources of these compounds. It has been demonstrated that N-acylethanolamines may be metabolized by lipoxygenase into oxylipins which could play a role in seed germination (Shrestha R et al., Plant Physiol 2002, 130, 391). A competition between fatty acid amide hydrolase and lipoxygenase has been reported in Arabidopsis seedlings (Kilaru A et al., J Biol Chem 2011, 286, 15205). It appears that these compounds have a variety of biological functions in plants such as defense signaling, cytoskeletal organization, but research is still at a relatively early stage. Thus, N-acylethanolamines are involved in flowering time control (Wang YS et al., PNAS 2006, 103, 12197). Overexpression of the Arabidopsis fatty acid amide hydrolase reduced N-acylethanolamines content and enhanced seedling growth, cell size and early flowering. It was also observed that an exogenous supplementation of these lipids delayed the onset of flowering (Teaster ND et al., Front Plant Sci 2012, 3:32).
Different targets, including the endocannabinoid system, may be involved in the immune-modulating activity of these derivatives.
Anandamide has been detected in truffles (Tuber melanosporum) at different maturation stages (about 6 pmol/mg protein) through liquid chromatography–mass spectrometric analysis (Pacioni G et al., Phytochemistry 2015, 110, 104). Its presence in truffles may be related to the poduction of melanin. As no endocannabinoid-binding receptors could be detected, the authors suggested that anandamide and its biosynthesis enzymes have evolved earlier than endocannabinoid-binding receptors, and that anandamide might be an ancient attractant to truffle eaters, that are well-equipped with endocannabinoid-binding receptors.
2 – Aminoalcohol linked as an ester to the fatty acid
O-arachidonoyl ethanolamine has its ethanolamine moiety linked as an ester instead of an amide to arachidonic acid, as in anandamide. It is known also as virodhamine.
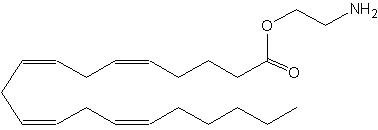
Virodhamine
It was isolated from human and rat brain and it was shown to have mixed agonist (CB1)/antagonist 5CB2) activity at the cannabinoid receptor (Porter AC et al., J Pharmacol Exp Ther 2002, 301, 1020). In rat brain the concentration of that compound was found to be similar to that of anandamide. Despite these properties, this lipid is likely the native endogenous agonist of the CB2 receptor and of the newly discovered GPR55 receptor (Pertwee RG et al., Brit J Pharmacol 2007, 152, 984).
Acyl urea are aminolipids produced by various fungi and may be considered as potent innate immune responses inducing attraction and activation of leukocytes (Schroder JM et al., J Biol Chem 2002, 277, 27887). Several symmetrical molecular species have been identified, they contain cis or trans palmitoleic, oleic or linoleic acid. The production of N,N’-all-cis-dipalmitoleyl urea (Fig. 2.29a) is the most potent response in inducing neutrophil chemotactic activity. These fungus-specific lipid mediator may thus alert the human innate immunity system to the presence of a fungal infection.

Diacyl urea
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire