
This family of simple lipids is mainly found as homologues of the most spread base sphingosine, a C18-aminodiol. Sphingosine was discovered around 1880 by Thudichum J but its structure was elucidated in 1947 (Carter HE et al., J Biol CHem 1947, 170, 285). Phytosphingosine is the counterpart of sphingosine in the plant world. This compound was isolated by Reindel in 1930 from yeast (Reindel F, Ann Chem 1930, 480, 76), and by 1940 its structure has been partially elucidated. Its distribution is not exclusively in plants since it was also detected in animal tissues in 1964 (Karlsson KA, Acta Chem Scand 1964, 18, 2397). A review gives an overview of the naturally occurring and synthetic sphingoid base-like compounds (Pruett ST et al., J Lipid Res 2008, 49, 1621).
All these amino alcohols occur largely in complex form (amides of fatty acids) as sphingolipids (ceramides, sphingomyelin, cerebrosides and complex glycolipids). More than 60 long-chain bases were described in bacteria, plants and animals with 12 to 20 carbon atoms , 2 to 3 hydroxy groups and zero to 2 double bonds, some may be phosphorylated or sulfated.
Complex poly amino alcohols with a long alkyl chain unrelated to sphingosine-type compounds are found in marine sponges.
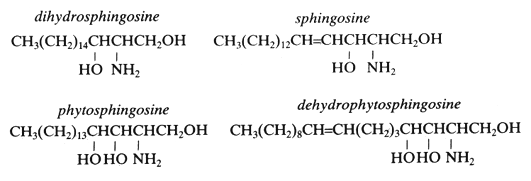
The simple amino alcohols are frequently represented by a simplified nomenclature similar to that used for fatty acids but with additional d or t to designate di- and trihydroxy bases, respectively. The most common amino alcohols are given in the table below:
|
Name |
Formula |
PM |
Chemical name |
| sphingosine d18:1 |
C18H37NO2 |
299 |
4-sphingenine (2D-aminooctadec-t-4-ene-1,3-diol) |
| dihydrosphingosine d18:0 |
C18H39NO2 |
301 |
sphinganine (2D-aminooctadecane-1,3-diol) |
| C20-dihydrosphingosine d20:0 |
C20H43NO2 |
329 |
eicosasphinganine (2D-aminoeicosane-1,3-diol) |
| phytosphingosine t18:0 |
C18H39NO3 |
317 |
4-hydroxysphinganine (2D-aminooctadecane-1,3,4-triol) |
| C20-phytosphingosine t20:0 |
C20H43NO3 |
345 |
4-hydroxyeicosasphinganine (2D-aminoeicosane-1,3,4-triol) |
| dehydrophytosphingosine t18:1 |
C18H37NO3 |
315 |
4-hydroxy-8-sphingenine (2D-aminooctadec-t-8-ene-1,3,4-triol) |
| sphingadienine d18:2 |
C18H35NO2 |
297 |
4,8-sphingadienine (2D-aminooctadeca-4,8-diene-1,3-diol) |
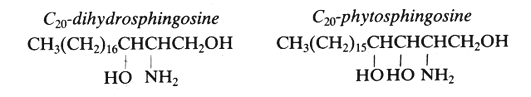
The initial step of the de novo biosynthesis of sphingosine is the condensation of serine with palmitoyl-CoA to form 3-ketodihydrosphingosine. This product is rapidly reduced by a NADPH-dependent reductase to dihydrosphingosine. Recent evidence suggests that this amine is first acylated by a fatty acyl-CoA to give a dihydroceramide which will be further converted to ceramide, the precursor of all sphingolipides, by the introduction of a trans-4,5-double bond. The demonstration of a differential regulation of that last step by palmitate versus oleate provides insight into the mechanisms of oleate-mediated protection against diabetes and metabolic disease (Hu W et al., J Biol Chem 2011, 286, 16596). Free sphingosine is only present in cell at very low concentrations since it is now considered as a lipid mediator. It was discovered that sphingosine (and other long-chain bases) inhibit protein kinase C in vitro and the common cellular responses to this enzyme (cell proliferation, platelet aggregation, neutrophile respiratory burst, differentiation of leukemic cells…) (review in : Hannun YA et al., Nature rev Mol Cell Biol 2008, 9, 139).
A review on this important properties appeared in Biochimica (Moscow). A review on the sphingoid bases biosynthesis has been released (Merrill AH, J Biol Chem 2002, 277, 25843).
Sphingadienine induces colon cancer cell death in vitro and prevent intestinal tumorigenesis in vivo (Fyrst et al., Cancer Res 2009, 69, 9457). Sphingadienines exert their influence by blocking Akt translocation from the cytosol to the membrane, thereby inhibiting protein translation and promoting apoptosis and autophagy. Thus, that compound may represent a new class of therapeutic and/or chemopreventive agents.
An analog of sphinganine (or dihydrosphingosine) but with 17 carbon atoms, present in bacteria-contaminated mussels, has been shown to be associated with a strong toxicity (diarrheic and paralysis symptoms) (Marrouchi R et al., Mar Drugs 2013, 11, 4724).
The accumulation of two neurotoxic amino alcohols, 1-deoxy-sphinganine and 1-deoxymethyl-sphinganine, were shown to be associated with an inherited sensory neuropathy (HSAN1) (Penno A et al., J Biol Chem 2010, 285, 11178). A mutation of the serine palmitoyltransferase induces a shift in its substrate specificity from serine to alanine or glycine. Both metabolites lack the C1 hydroxyl group of sphinganine. One of these compounds is shown below.
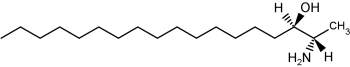
1-Deoxy-sphinganine
Two antifungal compounds, designated sphingofungin B and C, isolated from a culture of Aspergillus fumigatus were shown to have broad spectrum antifungal activity, but little or no antibacterial activity (Van Middlesworth et al., J Antibiot 1992, 45, 861).
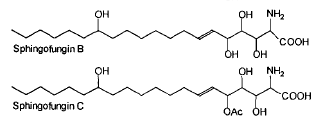
The structure of these compounds revealed a resemblance to the long chain bases found in sphingolipids. They may be described as amino fatty acids or carboxylated long chain base. This resemblance is in agreement with the demonstration of an inhibition of the first committed enzyme of sphingolipid biosynthesis, serine palmitoyltransferase (Zweerink MM et al., J Biol Chem 1992, 267, 25032).
Another amino fatty acid, myriocin, was discovered as an antibiotic from the culture broth of a fungus, Myriococcum albomyces (Klüpfel D et al., J Antibio 1972, 25, 109). Later, it was also isolated from the culture broth of Isaria sinclairii, a mushroom used in Chinese folk medicine which has potent immunosuppressive properties (Fujita T et al., J Antibiot 1994, 47:208 ).
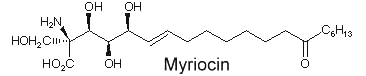
The chemical modification of myriocin (2-alkyl-2-aminopropane-1,3-diol) yielded a new compound, FTY720 (fingolimod), which was highly effective in experimental allotransplantations and against autoimmune diseases (multiple sclerosis, rheumatoid arthritis) (Chiba K, Pharmacol Ther 2005, 108, 308).
An anhydrophytosphingosine, with a furan group, named pachastrissamine, has been isolated as a cytotoxic principle of a marine sponge, Pachastrissa sp (Kuroda I et al., J Nat Prod 2002, 65, 1505). The tetrafuran group and the saturated chain likely derived from a phytosphingosine precursor.
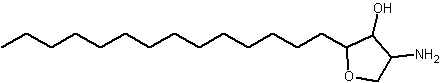
Pachastrissamine
Similar sphingoid bases have been isolated from another marine sponge, Penares sp (Ando H et al., J Nat Prod 2010, 73, 1947). They have unsaturated chain, methylated or not, of 24 or 26 carbon atoms. They exhibit moderate cytotoxicity against HeLa cells.
Sphingosine (and sphinganine) can be converted to sphingosine-1-phosphate (and sphinganine-1-phosphate) by a specific kinase. In 1970, Stoffel (Hoppe-Seyler’s Z Physiol Chem 1970, 351, 635) reported the formation of sphingosine-1-phosphate in erythrocytes, a kind of phosphosphingolipid which appeared later involved in cellular proliferation. It is classified here as simple lipid since formed of one component and a phosphate group. Sphingosine-1-phosphate is normally present in low abundance under normal physiological conditins (about O.1 mol% of total cell lipids). It was recently shown that this compound is rapidly produced in response to mitogenic concentrations of sphingosine. It can, at very low concentrations, induce increased DNA synthesis and cell division in some cells but is also a potent growth inhibitor for some human breast cancer cell lines. Many experiments have shown that sphingosine-1-phosphate is involved in the regulation of the traveling of lymphocytes T and B (lymphocyte egress) from thymus to peripheral lymphoid organs, an important step of the adaptive immunity (Matloubian M et al., Nature 2004, 427, 355). Curiously, important amounts of sphingosine-1-phosphate were detected in human platelets. Furthermore, mounting evidence indicates that multiple antiatherogenic or anti-inflammatory actions of lipoproteins HDL independent of cholesterol metabolism are mediated by the lipoprotein-associated sphingosine-1-phosphate through its receptors (Okajima F et al., Endocr J 2009, 56, 317). Further investigations indicate that HDL-associated sphingosine-1-phosphate is responsible for the beneficial effects of these lipoproteins on vasorelaxation, cell survival, cell adhesiveness, angiogenesis and synthesis of two powerful endogenous anti-atherogenic and anti-thrombotic molecules such as nitric oxide (NO) and prostacyclin (PGI2) (Rodriguez C et al., Thromb Haemost 2009, 101, 665). New data proposes S1P as an essential second messenger, whose synthesis is enhanced by photoreceptor trophic factors, such as GDNF (glial derived neurotrophic factor) and docosahexaenoic acid, in order to control key processes in the development of photoreceptors (Rotstein NP et al., J Lipid Res 2010, 51, 1247).
This compound can be cleaved to ethanolamine phosphate and t-2-hexadecanal by a pyridoxal phosphate lyase. This ethanolamine phosphate may be used for the synthesis of phospholipids such as phosphatidyl ethanolamine and phosphatidyl choline, the fatty aldehyde being used for the synthesis of plasmalogens or fatty acids.
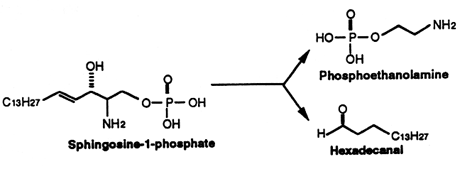
Sphinganine-1-phosphate (or dihydrosphingosine-1-phosphate) has also been found to bind to sphingosin-1-phosphate receptors, but its binding ability is less potent than that of its homologue (Tamama K et al., Biochem J 2001, 353, 139). That derivative has been shown to be involved in the stimulation of metalloproteinase 1 in association with tumor growth and metastasis (Bu SM et al., FASEB J 2006, 20, 184).
Comprehensive reviews of sphingosine-1-phosphate and other sphingolipid mediators have been released (Fyrst H et al., Nature Chem Biol 2010, 6, 489; Hannun YA et al., Nature rev Mol Cell Biol 2008, 9, 139) and its important regulatory properties can be found in Biochimica (Moscow) and in papers by Hla T (Sem Cell Develop Biol 2004, 15, 513) and by Takuwa Y (Biochim Biophys Acta 2008, 1781, 483) and Ksiazek M (J Lipid Res 2015, 56, 1271).
In plants, a role for sphingosine-1-phosphate has been shown in drought stress and stomacal guard cell closure, linking the phosphorylated lipid-derived signal to this calcium-mediated process (Ng C et al.,Nature 2001, 410, 596). Moreover, intracellular sphingosine-1-phosphate plays an important role as an intermediate of the sphingolipid-to-glycerophospholipid metabolic pathway (review in Kihara A, Biochim Biophys Acta 2014, 1841, 766).
Sphingosine sulfates have been isolated from a marine sponge (Spirastrella abata) and were shown to exhibit significant cytotoxicity against tumor cell lines (Alam N et al., J Nat Prod 2002, 65, 944). Two homologue structures were elucidated, the carbon chain having either 17 or 18 carbon atoms, the sulfate group at the C-4 position and the double bond being at the C-6 position (instead of the C-4 position in the sphingosine molecule).
An unusual sulfonated derivative of a 17-carbon amino alcohol was shown to be a major component of the cell envelope of gliding bacteria of the genus Cytophaga and of closely related genera (Godchaux W et al., J Bacteriol 1980, 144, 592). This lipid, named capnine, a structural analogue of sphinganine, was purified and was shown to be 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid. N-acylated derivatives of capnine were also described.
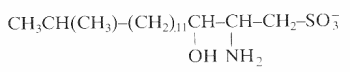
Similar sulfonolipids have been described in diatoms (Anderson R et al., Biochim Biophys Acta 1978, 528, 89).
A carboxylated capnine (halocapnine) is part of a ceramide analogues found in halophilic bacteria, the acylhalocapnines (Baronio M et al., J Lipid Res 2010, 51, 1878).
Branch-chain sphingoid bases have been described in some marine invertebrates. Thus, a base with a branched C19 alkyl chain and three double bonds, 2-amino-9-methyl-4,8,10-octadecatriene-1,2-diol, was shown to be present in glucosylceramide from starfish (Irie A et al., J Biochem 1990, 107, 578) and in sphingomyelin from squid nerve (Ohashi Y et al., J Lipid Res 2000, 41, 1118). A branched base with two double bonds has been found in cerebrosides from a sea anemone (Karlsson KA et al., Biochim Biophys Acta 1979, 574, 79) and from mycelia of a fungus (Kawai G et al., J Lipid Res 1985, 26, 338). The presence of these branched and unsaturated sphingoid bases in primitive organisms may indicate an essential role in their cell membranes.
While the glycosphingolipids in plants and animals share a sphingoid base as a common feature, a dimeric amino alcohol base was shown to be present in certain calcareous sponges. These compounds have a symmetrical, or almost symmetrical, long hydrocarbon chain (C28-C30) with at both ends a vicinal amino alcohol.
Two of these typical dimeric molecules, leucettamol A and B, have been described in a free state in a sponge from Micronesia Leucetta microraphis (Kong FH et al., J Org Chem 1993, 58, 970). One of these 2-amino-3-hydroxy hydrocarbons is shown below
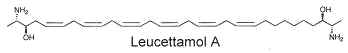
These molecules were shown to have only mild antibacterial activity. The absolute configuration of leucettamol A has been determined by precise physical methods (Dalisay DS et al., J Nat prod 2009, 72, 353).
Parent compounds containing a 1,3-diamino-2-propanol moiety, coriacenins, have been described in a Mediterranean sponge Clathrina coriacea (Casapullo A et al., J Org Chem 1996, 61, 7415).
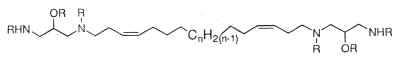
n = 9, 10 or 11; R = H or Ac
Another one, rhapsamine, was extracted in an Antarctic sponge Leucetta leptorhapsis (Jayatilake GS et al., Tetrahedron Lett 1997, 38, 43, 7507). It has the structure of a linear C28 polyene terminally substituted by 1,3-diaminoglycerol groups and has cytotoxic activity.
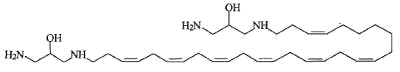
A related molecule has been described in an Australian calcareous sponge (class Calcarea) and named BRS1 (Willis RH et al., Toxicon 1997, 35, 1125).
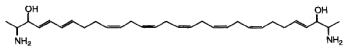
Others, crucigasterins, were described in a Mediterranean tunicate Pseudistoma crucigaster (Jares-Erijman EA et al., J Org Chem 1993, 58, 5732).
All these molecules were shown to have various cytotoxic activities. Furthermore, they can be glycosylated thus forming simple "sphingolipids" with potent cytotoxic properties.
CERAMIDES
Ceramides are amides of fatty acids with long-chain di- or trihydroxy bases, the commonest in animals being sphingosine and in plants phytosphingosine. The acyl group of ceramides is generally a long-chain saturated or monounsaturated fatty acid. The most frequent fatty acids found in animal ceramides are 18:0, 24:0 and 24:1(n-9), long-chain hydroxy fatty acids are also found. Some sulfonic acid derivatives of ceramides have been described and ceramide 1-sulfates have been isolated from marine Bryozoa.
Free ceramides have been found in small amounts in animal tissues (the stratum corneum of the skin is exceptionally rich in ceramides) and they are now considered as lipid messenger molecules with an emerging role in growth suppression and apoptosis (cell death) (Hannun YA et al., Nature Rev Mol Cell Biol 2008, 9, 139). Investigations have shown that ceramide is a critical mediator for triggering photoreceptor apoptosis in mammalian retina and suggest that modulating ceramide levels may provide a therapeutic tool for preventing photoreceptor death in neurodegenerative diseases. (German OL et al., Invest Ophthalmol Vis Sci 2006, 47, 1658). A dihydroceramide (sphinganine linked to 2-hydroxy-palmitic acid) isolated from Acetobacter, derived from vinegar, was shown to omprove cognitive function in dementia model rats (Fukami H et al., J Agric Food Chem 2010, 58, 4084).
A review of the important regulatory properties of ceramides can be found in Biochimica (Moscow) and a review of the roles for endogenous ceramide in mediating/regulating specific cellular responses may be consulted (Hannun YA et al., J Biol Chem 2002, 277, 25847). Chain length-specific actions of ceramides in different cellular processes and pathologies have been reviewed (Grosch S et al., Prog Lipid Res 2012, 51, 50).
Ceramides are the simplest sphingolipids and situated at the center of sphingolipid metabolism. Thus, the transfer of a phosphorylcholine head group from phosphatidylcholine to ceramide yields another phospholipid, sphingomyelin (also sphingolipid) and the addition of carbohydrate groups from the sugar donor, UDP-hexose, yields complex glycosphingolipids (cerebrosides, sulfatides, gangliosides). These compounds can be converted back to ceramide by the removal of sugars (glycosidases) or phosphorylcholine by sphingomyelinases. An enzyme (ceramidase) is able to cleave the amide-linked fatty acid of ceramide and free sphingosine.
Ceramides can be released from sphingomyelin or by de-novo synthesis in response to substances like vitamin D3, tumor necrosis factora, g-interferon, or interleukin 1. In most cell types, ceramides mediate antimitogenic responses such as cell differentiation, cell cycle arrest, cell senescence or apoptosis (Ruvolo PP, Pharmacol Res 2003, 47, 383).
Free ceramides are separated with the neutral lipid fraction on a silica column and the minute amounts present in cell extracts are best analyzed after derivatization before HPLC. Their chromatographic behavior is not very different from that of diacylglycerols which are structurally similar. The amide linkage is resistant to hydrolysis but is disrupted by prolonged heating in an alkaline medium but better with an acidic reagent. Ceramide can be prepared conveniently from complex glyco sphingolipides by chemical degradation.
Investigations on skin have revealed that ceramides play an important role in the formation of a water permeability barrier. That barrier, localized in the stratum corneum layer, is essential for mammalian terrestrial life, because it restricts transepidermal water loss in protecting animals from dessication (Uchida Y et al., J Dermatol Sci 2008, 51, 77). The molecular heterogeneity of ceramides synthesized in the epidermis and their possible roles in epidermal permeability barrier functions have been reviewed in 2009 (Mizutani Y et al., Biochimie 2009, 91, 784).
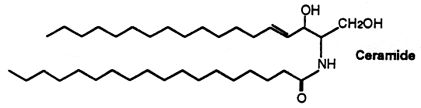
The stratum corneum of the skin (the upper-most layer made of dead cells and corneocytes) has a unique lipid composition which comprises mostly free fatty acids, cholesterol and ceramides (including O-acylceramides). These are different molecular species differing by the head group architecture and by the mean fatty acid chain length. The fatty acid esterified to the amide of the (phyto)sphingosine head group can be either a w-hydroxy or nonhydroxy fatty acid. The fatty acid chain length varies from 16 to 34 carbon atoms. One ceramide (figure below) contains linoleic acid linked to the long chain (n=30) w-hydroxy fatty acid. This molecule is thought to be of importance for proper skin barrier function (Bouwstra et al. J Lipid Res 1998, 39, 186).
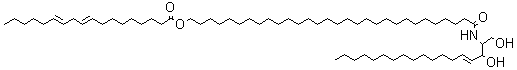
A new class of acylceramides, the 1-O-acylceramides, have been described in humans and mice epidermis. These compounds contain, in both the N- and 1-O – position, long to very long acyl chains (from C16 to C26). They make up about 5% of all esterified ceramides in epidermis. Considering their chemical structure and hydrophobicity, it has been postulated that they should contribute to the water barrier homeostasis in skin (Rabionet M et al., J Lipid Res 2013, 54, 3312).
These lipids have potential properties which are used in the treatment of various forms of dermatoses (Coderch L et al., Am J Clin Dermatol 2003, 4, 107).
It was shown that all the w-hydroxyceramides of corneocyte lipid envelopes are attached to proteins through their w-hydroxyl groups (Stewart ME et al., J Lipid Res 2001, 42, 1105). Thus, even after polar solvent extraction, the cells remain coated with a bound lipid monolayer, which can be released only by mild alkaline hydrolysis.
Another type of related compounds was detected in pig and human epidermis in 1978 by Gray GM et al. (Biochim Biophys Acta 1978, 528, 127). Its structure was elucidated later (Wertz PW et al. Prog Lipid Res 1986, 25, 383) and described as the glucosylated version of the O-acylceramide shown previously (O-acylglucosylceramide or glycosphingolipid), the glucose moiety being linked to the a-hydroxyl group of sphingosine.
A review may be consulted for the recent advances in our understanding of the synthesis and degradation pathways, physiological functions, and pathology of epidermal ceramides and acylceramides (Kihara A et al., Prog Lipid Res 2016, 63, 50).
An unusual ceramide (and its corresponding glucopyranoside derivative) with a C15 fatty acid linked to a C27 phytosphingosine-type amino alcohol was isolated from lipid extracts of a medicinal herb (Conyza canadensis, composita ) (Mukhtar N et al., Phytochemistry 2002, 61, 1005). The medicinal properties of this plant could be related to the presence of these sphingolipids which have been reported to exhibit antihepatotoxic and immunostimulatory activities (Kim et al., J Nat Prod 1997, 60, 274; Natori et al., Tetrahedron 1994, 50, 2771). A ceramide with a2-hydroxylated C24 fatty acid linked to a C18 sphingosine-type amino alcohol unsaturated at the carbon 11 has been isolated in leaves of Premna microphylla, a plant used in Chinese folk medicine (Zhan ZJ et al., Lipids 2003, 38, 1299).
A novel ceramide type has been described in bulbs of Zephyranthes candida (Amaryllidaceae), species used as ornamental and medicinal plant in China (Wu ZP et al., Lipids 2009, 44, 63). That ceramide, candidamide B, is characterized by a tertiary amide structure.
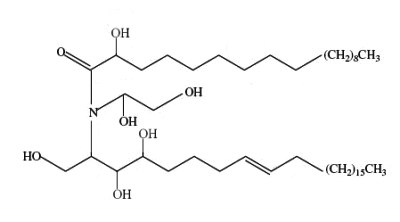
Candidamide B
Several ceramides were described in mushrooms. As an example, phytosphingosine-type ceramides formed of saturated dehydrophytosphingosine (2-amino-1,3,4-octadecanetriol) linked to various hydroxylated fatty acids (from 22 to 26 carbon atoms) were reported to be present in an edible Japanese mushroom (Grifola frondosa) (Yaoita Y et al., Chem Pharm Bull 2000, 48, 1356).
Novel ceramides named lactariamides were isolated from the fungus Lactarium volemus (Russullaceae) and determined to be formed from a hydroxylated fatty acid (2-hydroxytetracosanoic acid or 2-hydroxyoctadecanoic acid) linked to an amino alcohol either methylated (2-amino-9-methyl-4,8-octadecadiene-1,3-diol) or bearing an epoxy group (2-amino-3,4-epoxyoctadecan-1-ol) (Yue JM et al., J Nat Prod 2001, 64, 1246).
New ceramides formed from the same methylated amino alcohol, as described above, linked to normal or hydroxylated fatty acids of 14 to 18 carbon atoms have been described in several mushrooms belonging to various families (Yaoita Y et al., Chem Pharm Bull 2002, 50, 681).
Novel ceramides with cyclopropane-containing alkyl chains (gracilarioside and gracilamide) were isolated and characterized from the red alga Gracilaria asiatica (Sun Y et al., J Nat prod 2006, 69, 1488). If we except the description of cerebrosides with cyclopropane-containing chains in marine sponges, it is the first description of these structures in plants.
A ceramide analogue, complanine, an inflammation-inducing compound has been isolated from the marine fireworm (Eurythoe complanata, Polychaeta) (Nakamura K et al., Org Biomol Chem 2008, 6, 2058). Its structure could be characterized by a di-unsaturated aminoalcohol with 11 carbon atoms N-acylated with a GABA derivative, trimethylammonium-4 butanoic acid. The activation of PKC by complanine was hypothesized to be one of the molecular mechanisms by which E. complanata induced inflammation. Two other compounds of the complanine family were isolated from the same marine worm (Nakamura K et al., J Nat Prod 2010, 73, 303).
Bathymodiolamides, ceramide derivatives, have been isolated from a deep-sea hydrothermal vent mussel, Bathymodiolus thermophilus (Andrianasolo EH et al., J Nat Prod 2011, 74, 842). These compounds are formed of an aminopentane tetraol substituted with three fatty acids, two saturated (C12 and C16) and one unsaturated (C16:3n-4 or C11:2n-4). One of the two ceramides is shown below. Both are able to induce apoptosis in two cancer cell lines, HeLa (IC50 0.4-0.5 mM and MCF7 (breast cancer) (IC50 0.1- 0.2 mM). These compounds represent new chemical structures and may have potential in future drug discovery efforts.
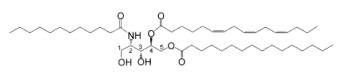
Bathymodiolamide A
Sulfonic-acid analogues of ceramides
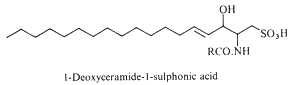
Deoxyceramide sulfonic acid : Marine diatoms (Nitzschia alba) were shown to contain a sulfonic acid derivative of a simple ceramide, N-acyl deoxysphingosine sulfonic acid, consisting of a 16-carbon fatty acid in amide linkage to sphingosine sulfonic acid (-CH2-SO3– replacing -CH2OH) (Anderson R et al., Biochim Biophys Acta 1978, 528, 77).
Capnoids (or N-fatty acyl capnine) are other sulfonic acid derivatives of ceramide which were first described in bacteria of the genus Capnocytophaga (Godchaux W et al., J Bacteriol 1980, 144, 592). Later, they were described in most gliding bacteria of the Cytophaga-like genera (they possess the ability to move over solid surfaces but not through liquids and possess no known locomotor organelles, such as flagella) (Godchaux W et al., J Biol Chem 1984, 259, 2982).
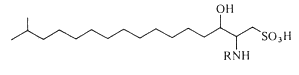
Capnoids are either capnine itself (R = H), which is 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid, or N-acetylated derivatives. N-acyl capnine was also found in the soil bacterium Chryseobacterium sp. and in a marine Flavobacterium sp. (Kamiyama T et al., J Antibiot 1995, 48, 929). Such compounds, also named sulfobacins, can also easily be synthesized (Sharma A et al., Tetrahedron Lett 2007, 48, 3705). These compounds have interesting properties since they inhibit the binding of von Willebrand Factor to its receptor with IC50S of 0.47 and 2.2 microM, respectively. Sulfobacin A also inhibits ristocetin-induced agglutination in human platelets fixed with paraformaldehyde with an IC50 of 0.58 microM (Kamiyama T et al., J Antibiot 1995, 48, 924).
The fatty acid methyl esters obtained from the lipids were heterogeneous (from 14, 15, and 16 carbon atoms), but in all cases were rich in hydroxylated fatty acyl groups, which constituted 66 to 95% of the total. The habitats range of these bacteria are from the human subgingival tooth surface (the Capnocytophaga spp.) to marine sediments (the marine Cytophaga sp.). The organisms of one genus (Capnocytophaga) are strictly fermentative (though aerotolerant), whereas the others are aerobes. A role in gliding motility of these sulfonolipids is suggested and an interest in this potential role is stimulated by the fact that the diatoms Nitzschia, the only other group of organisms of which at least one member contains N-acylaminosulfonates (see above), exhibit a form of motility which resembles that of the gliding bacteria.
A capnoid with a C14 hydroxylated and branched fatty acid was described at a high concentration (18% of polar lipids) in a Gram-negative, fresh-water, ring-forming bacterium Flectobacillus major (Batrakov SG et al., Biochim Biophys Acta 2000, 1484, 225). An analogue of the previous capnoid, but with a mono-unsaturated sphingoid base, has been described in a Gram-negative marine bacterium Cyclobacterium marinus (Batrakov SG et al., Biochim Biophys Acta 1998, 1391, 79).
Novel capnoids, variants of the previously described, have been found in extremely halophilic bacterium Salinibacter ruber (Corcelli A et al., Appl Environ Microbiol 2004, 70, 6678). They have have been shown to have the structure 2-carboxy-2-amino-3-O-(13′-methyltetradecanoyl)-4-hydroxy-18-methylnonadec-5ene-1-sulfonic acid. This sulfonolipid may be used as a chemotaxonomic marker for the detection of these bacteria.
The capnine analog (halocapnine) constituting the backbone of these sulfonolipids is slightly different from the capnine of Capnocytophaga and Flexibacter, as it corresponds to an iso branched unsaturated sphingoid base containing an additional hydroxyl group in C4 and an additional COO- group in C2 .To distinguish the capnine of the halophilic bacteria from those previously found in other organisms the name of halocapnine has been proposed (Baronio M et al., J Lipid Res 2010, 51, 1878).

Acylhalocapnine of Salinibacter ruber
R = iso C14 chain
Sulfate analogues of ceramides
For the first time two ceramide 1-sulfates have been isolated from the Bryozoa Watersipora cucullata (Ojika M et al., Tetrahedron Lett 1997, 38, 4235). One of these compounds is shown below.
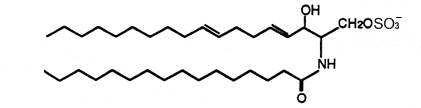
Ceramide 1-sulfate
The other one has a C18 fatty acid linked to a branched analogue of sphingatrienine. These compounds showed the inhibitory activity against a human topoisomerase I with IC50s of 0.2 to 0.4 mM.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire