
GLYCOSIDES OF FATTY ALCOHOLS
These alkyl glycosides are composed of a glycosyl moiety (one or several units) linked to the hydroxyl group of a fatty alcohol which may be a normal-chain, a branched-chain or a phenolic alcohol. Some important glycosides, the glycosyl phosphopolyprenols, are involved in several reactions leading to protein glycosylation.
Alkyl glycosides and polyglycosides
Alkyl glucosides have been described in heterocysts in Cyanobacteria where they likely take part in the protection of these cells. They were isolated and studied in nitrogen-fixing cyanobacteria such as Anabaena cylindrica (Nichols BW et al., Nature 1968, 217, 767) and several forms were structurally characterized (Lambein F et al., Biochemistry 1973, 12, 791). One of them is shown below.
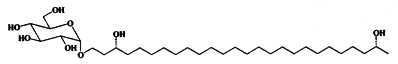
Other forms with different chain length and functional groups on the chain have been studied in other species (Cyanospira rippkae, Anamaeba torulosa). Their structures consist of sugar moitiess glycosidically bound to long-chain diols, triols, keto-ols and keto-diols. The aglycone moiety consisted of C26 or C28 carbon-chains with hydroxyl groups at the C-3, w-1 or w-3 positions. Keto-ols and keto-diols contained their carbonyl functionalities likely at the C-3 position.
Their formation and functions have been reviewed in 1999 (Adams DG et al., New Phytol 1999, 144, 3) and their distribution in 2009 (Bauersachs T et al., Phytochemistry 2009, 70, 2034).
Alkyl glucosides found in Nostocaceae were characterized by the presence of triols and of C-3 ketones.
Ascarosides are a group of simple glycolipids which were described in eggs and tissues of nematodes (Ascaridoidea) (Fouquey C et al., Bull Soc Chim Biol 1957, 39, 101). Investigations on the chemical nature of ascarosides from Parascaris equorum and Ascaris suum (Bartley JP et al., J Nat Prod 1996, 59, 921) have shown that they are formed basically by a glycosyl moiety (3,6-didesoxymannose, known as ascarylose) linked to a 2-hydroxylated hydrocarbon containing between 26 and 33 carbon atoms.
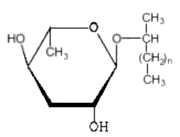
Ascarosides
n = 23 to 30
In both nematodes, species with another hydroxyl function in different positions along the hydrocarbon chain (diols) have been described. As these compounds are abundant in the internal lipid layer of nematode eggshells, thay are believed to be responsible for the chemical resistance of these eggs. It is noteworthy that ascarylose is also present in the lipopolysaccharide of several bacteria (Yersinia pseudotuberculosis, Pasteurella Pseudotuberculosis).
Other ascarosides are involved as pheromones in the control of the development of the worm Caenorhabditis elegans in response to high population or low food supply. These dauer pheromones are formed by a hydroxylated fatty acid linked to the same oligosaccharide, the ascarylose.
A group of polyacetylenic glucosides (bidensyneosides) has been described in a Chinese medicinal plant, Bidens parviflora (Asteraceae) (Wang NL et al., Chem Pharm Bull 2001, 49, 938). One form is shown below.
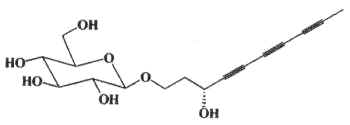
The glucose moiety is linked to an alkyl chain (from 10 to 14 carbon atoms) with 2 or 3 acetylenic bonds and with 1 or 2 hydroxyl groups. These compounds were shown to be potent inhibitors of histamine release and nitric oxide production.
A similar compound, cytopiloyne, has been isolated from the plant Bidens pilosa and has been reported to prevent type 1 diabetes in mice (Chang C et al., J Immunol 2007, 178, 6984). This glycolipid contains a diol with four triple bonds and 13 carbons. Experiments suggested that cytopiloyne prevents diabetes via T cell regulation.
Simplexides are unusual glycolipids which have been isolated from Caribbean marine sponges (Plakortis simplex) and are composed of a long-chain secondary alcohol ranging from C34 to C37 (which may be branched at one end) linked via the hydroxyl group to a disaccharide chain.
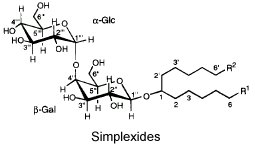
R1 and R2 form the two ends of the aliphatic chain with 17 to 19 carbon atoms each.
These compounds were shown to be immunosuppressive by a non-cytotoxic mechanism (Costantino V et al., Bioorg med Chem Lett 1999, 9, 271)
Alkyl glycosides are acetals obtained by condensation of glycosides with a fatty alcohol. In industry, saccharose, glucose, and sorbitol, which are available in large amounts, are used since 1977 as the starting raw carbohydrates with fatty alcohol for the synthesis of surfactants.
Alkyl polyglycosides have been known for a long time. Their synthesis was reported by Fischer in 1893 in reacting glucose and anhydrous ethanol the presence of HCl (Fischer E, Ber 1893, 26, 2400). The first patent describing the tensioactive properties of alkyl polyglycosides was registered in 1934.
Similarly, reaction of glucose with fatty alcohol produces alkyl glucosides; N-methylglucamides are prepared by reductive amination with methylamine and subsequent acylation. The alkyl glucosides are highly effective surfactants in washing and cleansing agents but are also widely used in the cosmetic products sector, as auxiliaries in crop protection formulations and as surfactants in industrial cleansing agents and today can already be said to be the most important sugar surfactants based on the yearly production amounts.
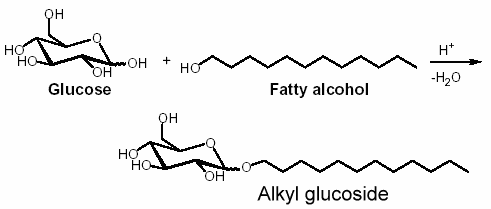
Only now, following several years of research work, it has been possible to develop reaction conditions that allow manufacture on a commercial scale. The structure on which these compounds are based corresponds exactly to the surfactant model. The hydrophobic (or lipophilic) hydrocarbon chain is formed by a fatty alcohol (dodecanol/tetradecanol) obtained from palm kernel oil or coconut oil. The tensioactive properties depend on the length of the carbon chain. The hydrophilic part of the molecule is based on glucose (dextrose) obtained from starch
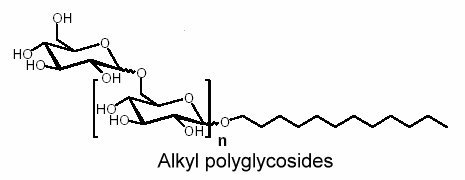
For example, the use of alkyl polyglycosides in a detergent or shampoo allows a reduction of the total amount of surfactants. In other combinations a particularly stable and fine foam can be produced which protects sensitive textiles. Alkyl polyglycosides have a good compatibility with the eyes, skin, and mucous membranes. On top of this they are completely biodegradable.
Prenol glycosides
Hydroxygeranyllinallol diterpenoid glycosides are abundant metabolites in three genera of Solanaceae ( Nicotiana, Capsicum, and Lycium) which have established antifeeding properties. These compounds consist of one or two acyclic geranyllinalool aglycones conjugated to one or more sugar moieties (Yahara S et al., Tetrahedron Lett 1988, 29, 1943).
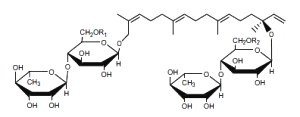
Hydroxygeranyllinallol diterpenoid glycoside
from Nicotiana obtusifolia
Several analogues have been isolated according to the chemical structure of R1 and R2.
Plakopolyprenoside
This cytotoxic glycolipid was extracted from the marine sponge Plakortis simplex and is composed of a C35 linear polyprenyl alcohol and a dixylosyl carbohydrate chain (Costantino V et al., Tetrahedron 2000, 56, 1393).
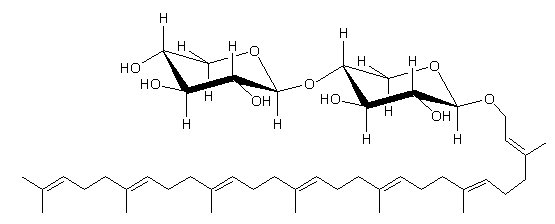
Plaxyloside, a compound similar to that described above, has a carbohydrate chain made up of six xylopyranose units and was also isolated from the same sponge species (Costantino V et al., Eur J Org Chem 2001, 23, 4457).
These simple phosphorylated glycolipids are not end products, as other glycolipids, but coenzymes which are concerned with the transfer of glycosyl residues from nucleoside diphosphate sugars to some appropriate acceptors, mainly proteins. In bacteria, they are involved in the formation of the complex polysaccharides of the cell wall while in eukaryotic cells they are involved in the protein N-glycosylation process. In yeasts and fungi these intermediates functions in protein O-mannosylation while in plants their role also encompasses the formation of glucans. In all cases they function as coenzymes of membrane-bound glycosyl transferases.
Both monophosphoryl and diphosphoryl derivatives of polyprenols have been determined in natural sources. Diphosphoryl polyprenols are mainly found as intermediates in the final assembly of oligosaccharide units in contrast to the monophosphoryl polyprenols concerned with monosaccharide transfer. Both types of glycosyl group exchange may be found in the same prokaryotic and eukaryotic systems.
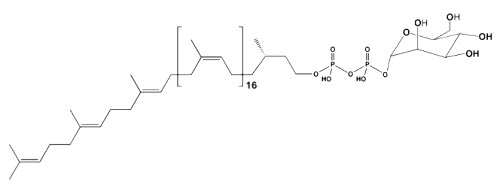
Mannosyl diphospho eicosaprenol
The lipid moiety of these glycolipids is formed in bacterial systems by polyisoprenoids containing 10 to 12 isoprenoid units, and in mammalian by dolichols containing 18 to 22 isoprenoid units. In plants, le lipid moiety is mainly formed by ficaprenols.
Phenolic glycolipids ("Mycosides")
In the fifties, Smith DW et al. (Am Rev Tuberc 1954, 69, 505) tried to characterize chemically immunizing fractions of extracts from Mycobacterium tuberculosis by infrared spectroscopy. This approach led to the discovery of the glycolipids called "mycosides" (Smith DW et al., Nature 1960, 186, 887). This heterogeneous family includes different structures such glycopeptidolipids and phenolglycolipids. According to the proposition of Smith DW et al. (Nature 1960, 186, 887), the later should be termed glycosides of phenolphthiocerol dimycocerosate. Structural works indicate they have an oligosaccharide moiety linked to a phenolphthiocerol molecule mainly esterified by mycoserosic acids, thus forming a kind of wax molecule related to the phthiocerol waxes (Fournié JJ et al., J Biol Chem 1987, 262, 3174).
Complex phenolic glycolipids belonging to the mycoside family have the general formula given below :
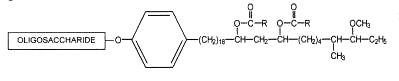
R = mycoserosic acid
Various forms have been described with various oligosaccharide residues and different fatty acid compositions.
Mycoside A was described in the Mycobacterium kansasii wall. The oligosaccharide moiety was shown to be : 2,6-dideoxy-4-methyl Arap-2-methyl acetyl Fucp-2-methylRhap-2-methyl-Rhap.
Mycoside B is characteristic of certain bovine strains of Mycobacterium tuberculosis. The oligosaccharide moiety was shown to have only one sugar component, a 2-O-methylrhamnose. Other phenolic glycosides with an oligosaccharide moiety consisting of methyl glucopyranose, and two molecules of methyl rhamnopyranose
Phenolic glycolipids are immunogenic with their carbohydrate at the non-reducing end. Thus, the knowledge of phenolic lipids has revolutionized the serological diagnosis of leprosy. The phenolic glycolipid from Mycobacterium leprae contains the unique determinant 3,6-di-O-Me-b-D-Glcp-2,3-di-0- Me-a-L-Rhap, which is highly reactive and specific for sera from lepromatous leprosy patients (Young DB et al., Science 1983, 221, 1057).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire