
Waxes is a general term used to refer to the mixture of long-chain apolar lipids forming a protective coating (cutin in the cuticle) on plant leaves and fruits but also in animals (wax of honeybee, cuticular lipids of insects, spermaceti of the sperm whale, skin lipids, uropygial glands of birds, depot fat of planktonic crustacea), algae, fungi and bacteria. Some waxes are of mineral origin. Montan wax originates from mob or lignite, that fossilized compound representing a late step of the transformation of vegetal into hydrocarbons.
The various materials named waxes do not form a chemically homogeneous group. All waxes are water-resistant materials made up of various substances including hydrocarbons (normal or branched alkanes and alkenes), ketones, diketones, primary and secondary alcohols, aldehydes, sterol esters, alkanoic acids, terpenes (squalene) and monoesters (wax esters), all with long or very long carbon chains (from 12 up to about 38 carbon atoms) and solid in a large range of temperature (fusion point between 60 and 100°C).
More commonly, waxes are esters of an alcohol other than glycerol (long chain alcohol, sterol, hydroxycarotenoids, vitamin A) and a long chain acid (wax esters). Wax esters are saponified by hot alkaline solutions and give a fatty acid and an alcohol. They are soluble in aromatic solvents, chloroform, ethers, esters and ketones.
R1 et R2 have commonly 10 to 20 carbon atoms or more. Example of wax esters are: dodecyl hexadecanoate (lauryl palmitate), octadecyl octadecanoate (stearyl stearate), etc. The acid or the alcohol chain can be unsaturated. In plants and some algae, phytol may be the alcohol component of was esters protecting leaves against dessication and parasites. Wax monoesters account for about 25% of sebum lipids in human, this wax being characterized by a high amount of an unusual fatty acid, 16:1n-10.
The physical characteristics of wax have maximized their usage in various industries, particularly in cosmetics. Wax are formulated in numerous personal care products due to their excellent emollient behavior. Wax esters are fine chemicals which are produced in low volume but are highly priced. Jojoba oil and sperm whale oil are natural waxes that fall within that fine chemicals group. Several attempts have been made to synthesize wax esters with cheap starting materials. Thus, waxes were synthesized from palm oil through enzymatic transesterification with oleyl alcohol using Lipozyme RM IM as the catalyst (Keng PS et al., Ind Crops Prod 2009, 29, 37).
In the uropygial gland of birds, the acids of wax esters may have a mono- or multi-branched chain (diester waxes). The physiological function of these waxy material is still a matter of debate but may contribute to protect birds against wetting, to make the feather flexible, to play a role as antiparasitic compounds or to provide UV protection. It has been shown that uropygial waxes shifted their structure during the mating season from a monoester type to one of two diester types.
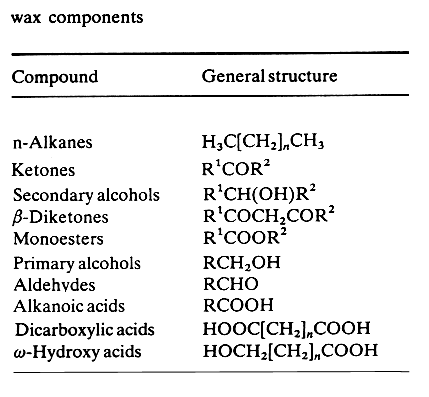
Waxes of type 1 are based on C8 to C16 b-hydroxy acids (R2) esterified with C6 to C16 fatty acid (R1) at the b-hydroxy position and with C16 to C20 fatty alcohol (R3) at the carboxyl group. These C30-C50 diester waxes have been described in several shorebirds (Rijpstra WI et al., J Nat Prod 2007, 70, 1804) and in female mallards (Anas platyrhynchus) (Kolattukudy PE et al., J Lipid Res 1987, 28, 582). Waxes of type 2 are based on C12-C23 alkane-1,2-diols (or 2,3-diols, sometimes named uropygiols) esterified with C10-C20 acids at the two hydroxyl groups. These diester waxes have been described first in chicken (Haahti EO e al. J Lipid Res 1967, 8, 131) and then in turkey (Hansen IA et al. J Lipid Res 1969, 10, 267), in the pheasant (Phasianus colchicus) (Saito K et al. J Biochem 1970, 67, 841) and in the red knot (Calidris canutus) (Sinninghe Damste JS et al., J Nat Prod 2000, 63, 381).
Various wax esters have been identified in the secretions of Meibomian glands (meibum) (Butovich I A et al., Lipids 2007, 42, 765). The three most abundant species were C18:1 fatty acid esters of C24:0, C25:0, and C26:0 fatty alcohol. Typically, a major was species based on C18:1 fatty acid and a saturated fatty alcohol was accompanied by a few related compound based on a C18:2, C18:3, and C18:4 fatty acid (Butovich IA et al., J Lipid Res 2009, 50, 2471). Meibum is an intrinsic part of the human tear film, the main role of which is to protect the ocular surface from dehydration. Among other proposed functions of the tear film are antimicrobial, lubricant, and nutritional ones.
Wax esters isolated from the mandibular canal of the porpoise (Tursiops gilli) are primarily (about 44%) isovaleryl (C-5) derivatives of fatty alcohols (Varanasi U et al., Biochemistry 1970, 9, 3629).That unique structure may be related to the function of the mandible lipid as a wave guide to the inner ear.
Polar zooplancton species are known for the storage of wax esters as natural energy reserves. Thus, in the antarctic Euphausiid Thysanoessa macrura the wax deposits reach up to 70% of the total body lipids and contain high levels of 18:1(n-9) and 18:1(n-7) alcohols (Kattner G et al., Mar Ecol Prog Ser 1996, 134, 295). Carnivorous zooplankton species are characterized by the presence of shorter-chain alcohols (14:0, 16:0) while herbivorous species, as the calanoid copepods, contain mainly long-chain alcohols (20:1, 22:1).
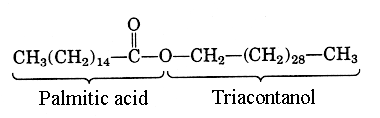
Triacontanylpalmitate is the main component of bee wax. Palmitic acid (C16:0) is esterified by a C30 chain, triacontanol (or melissyl alcohol).
Don’t forget that the word "wax" is derived from the old English "weax" for the honeycomb of the bee-hive. Thus, bee wax can be considered as the reference wax.
In plants the outer covering consists of an hydroxy fatty acid polymer called cutin. The underground parts and healed wound surfaces of plants are covered with an analogous substance, suberin. These substances are frequently mixed with other lipids and form a complex mixture called epicuticular wax. Cutin is a lipidic polymer containing C16 and C18 families of acids. The former is more abundant in growing parts, the later is present in the cuticle of slower-growing plants. These acids may be saturated, unsaturated, mono- or di-hydroxylated. In the cutin structure, a polyester structure exists where cross-linking depends on the availability of secondary hydroxyl groups. It has even been suggested that a biopolymer called "algaenan" consisting mainly of linear polyester chains cross-linked by ether bridges is present in cell wall of green microalgae (Blokker P et al. Phytochemistry 1998, 49, 691). In some microalgae (Chlorella, Scenedesmus, Tetraedron), the presence of complex ester waxes with long-chains (up to C80) have been described (Allard B et al., Phytochemistry 2001, 57, 459). In contrast, the major carbon chains of suberins are w-hydroxy acids and dicarboxylic acids, all with very long chains (>20 carbon atoms).
Among the least polar components of plant surface lipids hydrocarbons with the odd number carbon chains (C15 up to C33) are predominant. Aliphatic alcohols in the C20-C34 range are also widespread in plant surface lipids.
Detailed analyses revealed distinct differences between waxes on the petals and other plant parts. Thus, the petal wax was found to contain unusually high concentrations of C22 and C24 fatty acids and primary alcohols, much shorter than those in leaf and stem waxes (C28-C32 fatty acids) (Buschhaus C., et al., Plant Physiol 2015, 167, 80). It can be hypothesize that petal wax rich in C20 to C24 alcohols decreases the effectiveness of the transpiration barrier while being beneficial for plantpollinator interactions.
Fatty acid esters formed from a dicarboxylic acid (6 to 20 carbon atoms) esterified by two saturated di-alcoohol molecules (7 carbon atoms) have been isolated from dry excrements of Chinese rodent (Trogopterus xanthipes) (Yang NY et al., Lipids 2009, 44, 849). One of them, shown below has very potent anticoagulative properties. These results are in accordance with the traditional use of these excrements ("Feces Trogopterus") in traditional medicine for treatment of amenorrhea, menses pain and retained lochia.
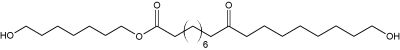
One of the fatty acid esters from the feces of Trogopterus
Pyrethrins are natural or synthetic organic compounds that have potent insecticidal activity. All are formed from a cyclopropane fatty acid esterified by an allylic alcohol having a cyclopentene core. The major natural forms are pyrethrin I and II. They differ by the structure of the group R of the acid moiety.
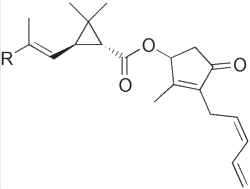
Pyrethrin I : R = CH3
Pyrethrin II : R = CO2CH3
The pyrethrins are contained in all parts of several species of Chrysanthemum were they were first discovered. Now, all pyrethrins are synthesized by chemists from various precursors.
3-MCPD fatty acid esters may be formed from 3-monochloropropane-1,2-diol during the production of fats and oils when they are heated with salt to high temperatures. Significant amounts of 3-MCPD fatty acid esters have been detected in numerous edible fats and fat-containing foods, for instance in infant formula. 3-MCPD esters have been found in all refined vegetable oils. The lowest levels were observed in refined rapeseed oil (0.31.5 mg/kg) and the highest levels in refined palm oil (4.513 mg/kg). No toxicological data are available on 3-MCPD esters.
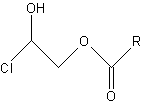
3-MCPD esters
Glycidol esters are present in refined oils. These esters are formed from added glycidol (stabilizer) and fatty acids when heated at high temperature. The fate of glycidol-esters in the human digestive tract remains unknown. Glycidol itselfs is probably carcinogenic to humans, comparable to acrylamide.
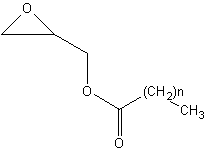
Glycidol esters
As their levels are very low in natural oils, very efficient methods must be used (Shimizu M et al., J Oleo Sci 2010, 59, 535).
As several compounds containing the allyl group (H2C=CH-CH2R) have a high activity as insecticides, acaricides, and insect repellents, allyl esters of fatty acids were synthesized. As an example, allyl esters of fatty acids have been proposed as wood preservatives, allyl pentanoate having the highest activity on termites (Yoshida S et al., Jpn Patent JP 08133909, 2006). Allyl esters of fatty acids (from 3 to 17 carbon atoms) have been synthesized. All have ovicidal effect on an insect (Cydia pomonella), the activity being inversely related to the alyl chain length (Escriba M et al., J Agric Food Chem 2009, 57, 4849).
In bacteria, Mycobacterium spp, characteristic waxes (phthiocerol waxes) were described in the wall cell lipids. These waxes are diesters of the phthiocerols, including phthiodiolone and phthiotriol, with mycocerosic acids. Thus, they are named dimycocerosate esters.
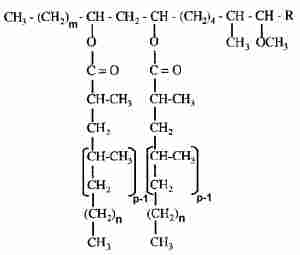
m = 20, 22
n = 16, 18
p = 2-5
R = CH2-CH3 or CH3
These waxes were shown to play a role in the virulence of the cell but also in its architecture and permeability (Camacho LR et al., J Biol Chem 2001, 276, 19845; Onwueme KC et al., Prog Lipid Res 2005, 44, 259).
A review on waxes may be found in the book published in "The Oily Press" by Hamilton RJ (Waxes: chemistry, molecular biology and functions, 1995).
Waxes are largely used in cosmetics, in pharmaceutical industries, as lubricants, polishes, plasticizers and in food industries. As natural sources of waxes are expensive and limited in access, they are frequently synthesized by enzymatic (Hallberg ML et al., JAOCS 1999, 76, 183) or chemical procedures (Aracil J et al., Zeolites 1992, 12, 233).
We give below a description of some waxes of industrial importance.
Bee wax
This wax is an abdominal secretion of bees (Apis mellifera), its colour being dependent of the flowers gathered by these insects. Bees used it to form the hive cells. Bee wax is easily saponifiable and emulsifiable because of its content in free fatty acids, diols and hydroxyacids.
Its main components are palmitate, palmitoleate, hydroxypalmitate and oleate esters of long-chain alcohols (C30-32) (about 70 to 80% of the total weight). The ratio of triacontanylpalmitate (or melissylpalmitate, C30 alcohol esterified by C16 fatty acid) to cerotic acid (C26:0), the other major component of bee wax is 6:1. Ethyl esters are also present, the most abundant species being ethyl palmitate, ethyl tetracosanoate, and ethyl oleate (Jimenez JJ et al., J Chromatogr A 2004, 1024, 147). Aliphatic hydrocarbons (from 10 to 18 % of heptacosane and nonacosane and other species from 17 up to 35 carbon atoms), unsaturated hydrocarbons from 21 up to 35 carbon atoms with one or two double bonds, sterols (up to 2% as cholesterol, lanosterol, b-sitosterol), pheromones (geraniol, farnesol) and terpenoids are also found. Its melting point is 62-65°C. A chemometric method based on the use of high-temperature gas chromatography has been reported for the detection of bee wax adulteration with mineral paraffin (Maia M et al., Food Chem 2013, 136, 961).
Bee wax is used since ancient times since its presence was detected in the wall pictures of the Lascaux cave and in Egyptian mummies. Ancient Egyptians used it also for its adhesive and coating properties, and in shipbuilding. In the Roman period, bee wax was used as a waterproofing agent and treatment for painted walls (medium for the "Fayum portraits"). In the Middle Ages, this wax was valuable and exchanged as a form of currency. In recent times, bee wax was used as a modeling material, as component of seals, coatings, polishes, and candles.
The world production amounts to about 7000 tons per year and 60% are used in cosmetic and pharmacy.
This wax is secreted by insects (Coccus ceriferus) and laid on tree branches (1500 insects are needed to produce 1g chinese wax). That insect is cultivated in China. Besides an important content in esters (about 83%), this wax includes some free acids, alcohols (up to 1%) and hydrocarbons (2 to 3%). Chemically, the esters are formed of chains with 46 up to 60 carbon atoms, the majority of alcohols and acids having 26 or 28 carbon atoms. The purified wax is used to make candles and polish.
Shellac wax
This wax (known also as lac wax) is produced by a cochineal insect (Tachardia lacca) native of India. It contains a majority of fatty esters (70-82%), free fatty alcohols (8-14%), acids (1-4%) and hydrocarbons (1-6%). The esters are formed of chains of 28 up to 34 carbon atoms.
This wax is used in varnish industry and may replace carnauba wax.
Spermaceti is extracted by cooling (11% of the initial oil) from adipose tissues and also collected from a big cavity in the head of a cachalot (Physeter macrocephalus) known as sperm whale. The frontal organ, used as a sonar by the animal, contains about 3 tons spermaceti for a 15 meters animal. This product contains fatty esters (65-95%) but also triglycerides (5-30%), free alcohols (1-5%) and acids (0-3%). Adipose tissues (9-10 tons of lard for a 15 m animal) contain only 10-12% spermaceti wax. Fatty esters are formed essentially of cetyl palmitate (C32) and cetyl myristate (C30). Purified spermaceti has an aspect of a light mass of white crystals which can be powdered. It can be fused with bee wax and other fatty compounds (oils, fatty acids). Its melting point is 42-50°C.
Spermaceti was used in medicine in England (15th century) and later in cosmetics, pharmacy and also in candles but, after the recent international regulation concerning whale captures, it is no longer produced and sold. It is now replaced by synthetic spermaceti made of pure cetyl palmitate or mixtures based on jojoba.
This material is secreted by sheep sebaceous glands and collected from crude wool by dilute alkali or detergent washing. Unwashed wool contains about 10-24% of greasy matter and a small proportion of salts of long-chain fatty acids. Lanolin contains fatty esters (14-24%), sterols and triterpene alcohol esters (45-65%), free alcohols (6-20%), sterols (cholesterol, lanosterol) and terpenes (4-5%). Hydroxylated fatty acids (mainly hydroxy palmitate) are found either free or esterified. Fatty acid chains have from 14 up to 35 carbon atoms, many of them having branched chains (iso or anteiso conformations). Its melting point is 35-42°C. The crude lanolin contains about 17% of primary alcohols and 9% of diols. Among monoalcohols, 9% have a normal chain, 38% belong to the iso series and 53% to the anteiso series. Two third of the diols belong to the iso series (Fawaz F et al., Ann Pharm Fr 1974, 32, 215). Among acids, 27% are a-hydroxylated, 5.2% are w-hydroxylated and 4.7% are poly-hydroxylated (Fawaz F et al., Ann Pharm Fr 1974, 32, 59).
As bee wax, lanolin is used since very ancient times in cosmetic and dermatology but is actually used in industry (fabric, ink, lubricant).
Vegetal waxes
Carnauba wax
This wax (known as "queen of waxes") is secreted by leaves of a Brasilian palm tree (Copernicia prunifera cerifera), about 100 g for one tree in a year. It contains mainly fatty esters (80-85%), free alcohols (10-15%), acids (3-6%) and hydrocarbons (1-3%). As a peculiarity, carnauba wax contains esterified fatty dialcohols (diols, about 20%), hydroxylated fatty acids (about 6%) and cinnamic acid (about 10%). This last phenolic acid compound (antioxidant in free form) may be hydroxylated or methoxylated.
This wax is the hardest and highest melting of the natural waxes (melting point : 78-85°C) and is used mainly mixed to bee wax to make various polishes for shoes, floor and furniture but also in cosmetics (lipsticks, creams) and in food industry (glazes for candies, gums, fruit coatings …). It is used also in the paper industry for paper coating (the largest application in the USA).
Ouricouri wax
It was first exported from Brazil in 1937 but has fallen in use in recent times.
It was extracted from the ouricouri palm (Syagrus coronata, Cocos coronata) by sraping the wax from the leaf surface. Its melting point is 81-84°C. Ouricouri resembles carnauba wax in its physical properties, thus, it was used as substitute in carbon paper inks, mould release lubricants and polishes.
Jojoba oil
This product resulted from the pressure on governments to replace spermaceti.
This wax is fluid (melting point: about 7°C) and produced by pressing from seeds of the jojoba tree (Simmondsia chinensis, Euphorbiacae), now cultivated in Mexico (Sonora), Arizona and California. The cultivation of jojoba is also experimented in Israel, Africa, Australia, and China.
It is formed quite exclusively of alcohols esterified with long-chain fatty acids (more than 98%) with a total of 38 to 44 carbon atoms. The fatty acids are 18:1n-9 (about 10%), 20:1n-9 (about 70%) and 22:1n-9 (15-20%), while the fatty alcohols have predominantly 20 and 22 carbon atoms and one double bond.
Jojoba oil is very resistant to oxidation and is largely used in cosmetic applications (soaps, shampoos, skin cream, anti-solar oils). Industries use sulfonated or hydrogenated oil as lubricant, polishes, candles and coatings. Future uses could be as foam control agent and low-calorie food additive.
Candelilla wax
This wax is produced by small shrubs from Mexico, Euphorbia cerifera and E. antisyphilitica (Euphorbiaceae). The wax is extracted by boiling the plant (to separate the wax and the plant material). The wax floats to the top of the water and is skimmed off and processed. It contains hydrocarbons (about 50% of C29 to C33, mainly C31), esters (28-29%), alcohols, free fatty acids (7-9%), and resins (12-14% triterpenoid esters). Its melting point is 67-79°C. It has been used mainly mixed with other waxes to harden them without raising the melting point. This wax is used in cosmetics (lip balms and lotion bars), pharmaceutics and in food stuffs (E 902, GRAS) to improve stability and texture as a substitute to beeswax (melting point : 66-71°C). One of candelilla’s major outlets was a binder for chewing gums.
Esparto wax
This wax is a by-product in the artisanal preparation of paper from a reed known in northwest Africa and southern Spain as "Halfah grass", Stipa tenacissima, it melt at 73°C. While its composition is highly variable, it contains hydrocarbons, esters, alcohol (C28) and triterpenoids.
Japan wax
That product is not a true wax but is more like a vegetable tallow found in the kernel and outer skin of the berries of Rhus and Toxicodendron species, including those yielding Japanese lacquer. It contains a high amount of palmitic acid triglycerides (93-97%), long chain dicarboxylic acids including C22 and C23 chains (4-5.5%) and free alcohols (12-1.6%). Its melting point is 45-53°C. That wax is much used in Japan in cosmetics, ointments and to make candles but becomes rancid with age.
Rice bran oil
Rice bran from the milling of rice, Oryza sativa, contains a wax mixed with triglycerides. The melting point of the pure wax is 75-80°C. It contains esters of fatty acids (26 to 30 carbon atoms) and long-chain alcohols (C26 to C30) and a large amount of unsaponifiable matter (55-67%).
That wax is much used as a constituent of chocolate enrobers, various fruit and vegetable coating and as a lipstick.
Mineral waxes
Ozocerite (or ozokerite)
This wax is found in lignite beds in Galicia in the Carpathian mountains, Russia, Iran, and United States (Utah). Most ozocerite consists of hydrocarbons (C20-C32) and its melting point is about 90°C. It is used in making lubricants, lipsticks, deodorants, polishes, and adhesives.
Montan wax
This wax is derived by solvent extraction of lignite or brown coal (sub-bituminous coal). As it has been preserved in the coal it is really fossilized plant wax. Thus, it has many characteristics similar to those of vegetal waxes. The earliest production on a commercial scale was in Germany during the latter half of the nineteenth century, and Germany continues to supply the majority of the worlds production of Montan wax. The composition of Montan wax depends on the material from which it is extracted, but all contain varying amounts of wax, resin, and asphalt. Resins must be removed by extraction with solvents (diethyl ether, acetone). The wax component of Montan is a mixture of long. chain (C24-C30) esters (62-68 wt %), long-chain acids (22-26 wt %), and long. chain alcohols, ketones, and hydrocarbons (7-15 wt %). Montan wax is hard and is one of the most resistant to oxidation. Carbon papers were the largest consumer of crude Montan wax. The highest present part (30%) of Montan wax is used in car polishes. Additional applications are shoe polishes, electrical insulators, and lubricant in plastics and in paper industry.
As natural waxes are versatile, they can suffer inherent variability in quality and availability, cosmetic product include more and more frequently synthetic waxes. They are made of ethylene glycol diesters or triesters of long-chain fatty acids (C18-C36). Their melting points range between 60-75°C and can be used to confer rigidity to sticks and to modify the product’s crystallinity.
While having the structure of waxes, esters of alcohols and fatty acids either with a straight or branched chain, but shorter than for waxes, are manufactured for cosmetic applications. Depending on the chain length and structural arrangement of the two starting materials, esters are tailored to provide different physical properties and types of emolience.
Straight chain esters, such as cetyl palmitate and cetostearyl stearate, which are solid at room temperature, are used to increase the viscosity of emulsions. Liquid branched chain esters, such as isopropyl myristate or cetostearyl ethylhexanoate, provide products with good spreading properties. Furthermore, the choice of the ester influences both the solubility and spreadability of sunscreen agents and their ability to penetrate the skin.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire