
![]()
PLASTOQUINONES
A substituted quinone was isolated in a lipid extract of lucerne by Kofler in 1946. The discovery of ubiquinone and of its role in mitochondrial electron transport stimulated studies of related substances. Crane FL (1959) suggested that this quinone was called plastoquinone because it appeared concentrated in the chloroplasts of higher plants. Rapidly, it appeared that plastoquinone plays a role analogous to that of ubiquinone in mitochondria but associated with the transformation of light into chemical energy. They are also present in algae and cyanobacteria.
The elucidation of the structure of plastoquinone was that of a 2,3-dimethyl-1,4-benzoquinone with a C45 side chain related to the isoprenoid alcohol, solanesol.
There are several plastoquinones with side chains of different length in position 5. They are designated as plastoquinone-n where n is the number of carbon atoms in the side chain or better as PQ-n where n indicates the number of isoprenoid units. n varies from 6 to 9.
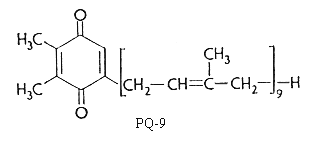
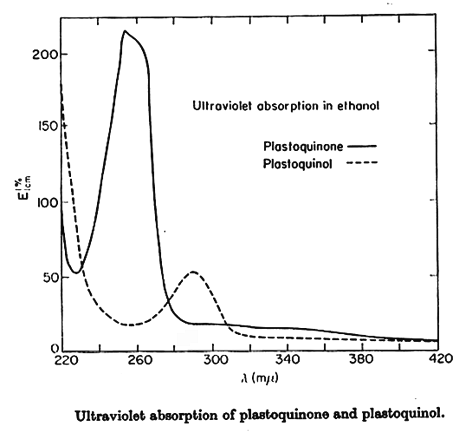
Plastoquinone is a yellow crystalline solid (PQ-9 has a MW of 748) soluble in most organic solvents. It has a characteristic UV spectrum with a maximum at 255 nm (molar abs. coeff.: 15700). On reduction (with sodium borohydride) a single maximum of lower intensity at 290 nm (molar abs. coeff.: 3440) is observed.
Several plastoquinone and plastohydroquinone analogues have been described in brown algae (Reddy P et al., Phytochemistry 2009, 70, 250). They differ primarily in the linear terpene chain moiety which has hydroxy and carbonyl groups in various positions. As an example, we give below the structures of the simplest (sargaquinone) and one of the most complex (fallaquinone) described in Sargassum fallax.
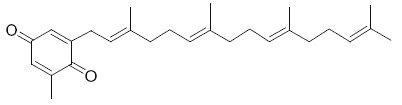
Sargaquinone
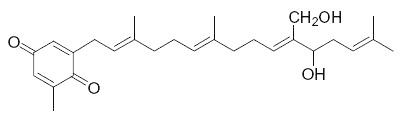
Fallaquinone
Many of these molecules displayed significant antioxidant, potent antiviral or moderate antitumour activities.
Various heterocyclic quinones have been described in sulphur-oxidizing archaebacteria, the benzothiophenoquinones.
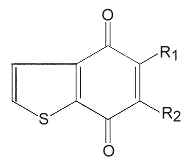
Several species of these quinones are present with menaquinones in the same bacteria. R1 may be -CH3 or -SCH3, and R2 is an isoprenoid chain , saturated or mono6unsaturated, with 3 to 6 isoprenoid units.
Several alkylated hydroquinones have been described in vegetals.
As an example, we give below the common structure of cytotoxic compounds discovered in Tapirira guianensis, a tall tree from Mexico to Brazil, used as folk medicine (m = 8 and n = 5) (David JM et al., J Nat Prod 1998, 61, 287) and in Lannea welwitschii (lanneaquinol with m = 6 and n = 7) (Groweiss A et al., J Nat prod 1997, 60, 116).
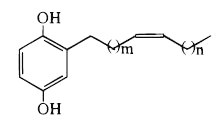
These two products displayed cytotoxic activity against human prostate cancer.
A large number of linear polyprenylhydroquinones (plastoquinones without methyl groups) have been isolated from sponges, especially from the Irciniidae family. Biological studies conducted on several of these molecules showed them to have a moderate antibacterial, antiviral, and anti-inflammatory activity. These linear polyprenylhydroquinones could be further divided in two main groups with polyprenylhydroquinones sulfates (De Rosa S et al., J Nat Prod 1995, 58, 1450) and hydroxylated polyprenylhydroquinones (Abed C et al., Mar Drugs 2011, 9, 1210).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire