
MONOTERPENES
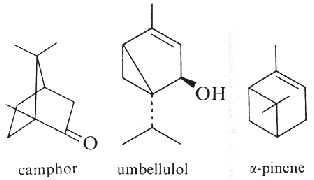
They are the terpenes that have been known for several centuries as components of the fragrant oils obtained from leaves, flowers and fruits. Monoterpenes, with sesquiterpenes, are the main constituents of essential oils. While a few, such as camphor, occur in a near pure form, most occur as complex mixtures, often of isomers difficult to separate. These essential oils have numerous actions, such as allelochemical functions between plants and between plants and predators. A role in wound healing was also observed.
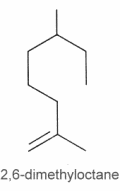
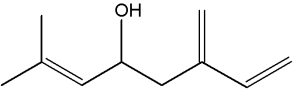
They can be considered as derivatives of 2,6-dimethyloctane.
Among natural molecules, the followings are well known and have several structural isomers.
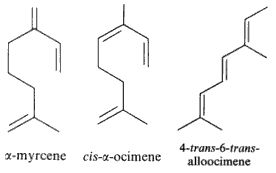
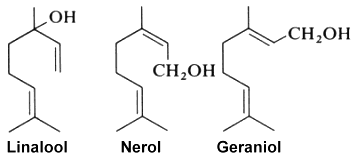
Defensive role of simple terpenes have been demonstrated as for more complex compounds. Ocimene and linalool (with farnesene) were shown to be produced by de novo biosynthesis in plants damaged by insect herbivories (Paré PW et al., Plant Physiol 1997, 114, 1161). These compounds likely mediate the interaction between herbivores and their natural enemies, attracted by terpenes. Experimentally, a study of Arabidopsis thaliana engineered to overexpress a terpene synthase leading to the emition of large amounts of linalool, which is normally produced only in trace levels. Compared with wild-type A. thaliana, the transgenic plants significantly repelled Myzus persicae aphids (Aharoni A et al., Plant Cell 2003, 15, 2866). That approach may be a solution to the protection of cultivated plants against insect attacks. Recent studies reveal a complex interplay between pollinator attraction and plant defense mediated by linalool and its derivatives. The diverse functions of linalool, ranging from toxin to long distance pollinator attractant are discussed in a review article (Raguso RA et al., Curr Op Plant Biol 2016, 32, 31).
Geraniol, produced by geranium, rose and lemon, has been determined to be also an alarm pheromone of the insect Corythucha ciliata which attacks the sycamore tree (Kuwahara Y et al., J Chem Ecol 2011, 37, 1211).
A great number of orchid species in the New World tropics have coevolved with the insects Euglossini by producing floral scents, highly attractive to insect males over great distances, for efficient pollination. Ipsdienol has been shown to be the main attractive component of the orchid scent (Schorkopf DL et al., J Chem Ecol 2011, 37, 953).
Ipsdienol
They are derived from cyclohexane with an isopropyl substituent. The most typical are :
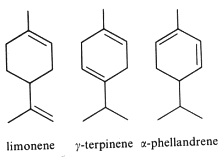
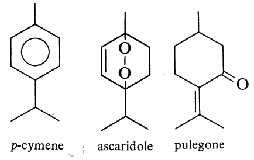
Limonene is an important volatile emitted by the holm oak (Quercus ilex), and acts as allelochemical in inhibiting seed germination of other plant species (Singh HP et al., Ann Bot 2006, 98, 1261). About 30,000 tons/year of limonene are extracted from natural sources (turpentine oil) and are used for the syntheses of other optically active products (Schwab W et al., Eur J Lipid Sci Technol 2013, 115, 3).
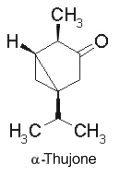
Thuyone is best known for being a toxic chemical (the a form is the most active) in absinthe, a product extract from Artemisia absinthium. Its psychedelic effects consecutive to absinthe consumption is disputed. Pharmacologically, thujone acts mainly on the GABA receptors in the brain and exhibits psychoactive response. In many countries the amount of thujone allowed in food or drink products is regulated (in Europe, the maximum level tolerated is 25 mg/l). Other plants containing thujone, such as the coniferous Thuja occidentalis, are used in herbal medicine, mainly for their immune-system stimulating effects.
Pinene is, as limonene, an allelochemichal emitted by the roots of Quercus ilex. Camphor and pinene are also allelochemicals emitted by Salvia leucophylla (Nishida N et al., J Chem Ecol 2005, 31, 1187). Pinene is among the more readily available optically active substance (about 30,000 tons/year) and is used for the syntheses of other chemical products (Schwab W et al., Eur J Lipid Sci Technol 2013, 115, 3).
Iridoids are a class of bicyclic monoterpenes found in a wide variety of plants and in some animals. They are often intermediates in the biosynthesis of alkaloids. Chemically, iridoids usually consist of a cyclopentane ring fused to a six-membered oxygen heterocycle, as exemplified by nepetalactone, the active ingredient in catnip (Nepeta spp), plant known for the behavioral effects they have on cats.
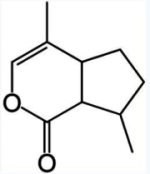
Nepetalactone
Iridoids are typically found in plants as glycosides, most often bound to glucose.
Cleavage of a bond in the cyclopentane ring gives rise to a subclass known as seco-iridoids.
Iridoids are found in many medicinal plants and may be responsible for the some of their pharmaceutical activities. Isolated and purified, iridoids exhibit a wide range of bioactivities including cardiovascular, hypoglycemic, anti-inflammatory, antispasmodic, antitumor, antiviral, immunomodulator and purgative activities (Didna B et al., Chem Pharm Bull 2007, 55, 159). They are produced by plants primarily as a defense against herbivores or against infection by microorganisms. To humans and other mammals, they have also a deterrent bitter taste. It can also be used as a mosquito repellent. As other terpenes, iridoids may function as protective substances in the animal kingdom, especially for insects. These compounds are obtained from the diet or, as the iridoids of leaf beetles, they are made by the insect itself.
Many monoterpenes possess antitumor activity in animal and cell models. They have also antioxidant properties, g-terpene and hydroxytyrosol being among the most effective.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire