
These lipids (known also as diglycerides) are fatty acid diesters of glycerol and occur in two isomeric forms:
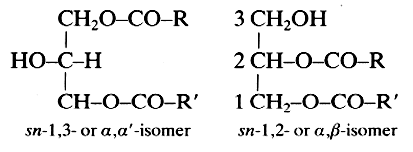
R and R’ are saturated or unsaturated hydrocarbon chains.
Diacylglycerols are generated mainly during digestion in the stomach and in the duodenal part of the intestine. In man, the lingual lipase preferentially hydrolyzes the ester bond in the sn-3 position of the triacylglycerols, thus generating sn-1,2-diacylglycerols. These molecules are isomerized in acidic solution with generation of sn-1,3-diacylglycerols which again are hydrolyzed in monoacylglycerols commonly found in the stomach content. In the duodenum as in the test tube, the lipase hydrolyzes easily the triacylglycerol ester bonds at the sn-1 and sn-3 positions giving a mixture of sn-2,3 and sn-1,2-diacylglycerols which again undergo chemical isomerization. In vitro, the isomerization is subject to both acid and base catalysis, and is particularly troublesome in the preparation of 1,2-diacylglycerols.
This process is used to do stereospecific analyses of triacylglycerols. More accurately, a non specific procedure is now used to generate diacylglycerols: the reaction with the Grignard reagent ethyl magnesium bromide which give all the possible combinations of diacylglycerols.
The abiotic synthesis of diacylglycerols has been shown to be possible in the laboratory under simulated hydrothermal conditions (Rushdi AI et al., Orig Life Evol Biosph 2006, 36, 93). These results indicate that condensation reactions under these primitive physical conditions may be the source of compounds for abiotic membranes which are candidates for micelle/vesicle formation at the beginning of life.
In cell biology, diacylglycerols are generated from phospholipids, the acyl chains being in the sn-1 and 2 positions. In lipid extracts, sn-1,3-diacylglycerols are always present, even in lower amounts than the other form, because of an artefactual isomerisation during isolation and purification. In cell extracts, diacylglycerol molecules have generally two different fatty acid chains, the most unsaturated being found at the sn-2 position.
Diacylglycerols are important intermediates in the biosynthesis of triacylglycerols and phospholipids and play a fundamental role in the cellular signaling. They are able to activate cellular mechanisms directly via protein activation or indirectly via the liberation of fatty acids which may be metabolized in agonist molecules (eicosanoids). The most famous molecular species of diacylglycerol generated by phospholipase C from phosphatidyl inositol is 1-stearoyl-2-arachidonoyl-sn-glycerol. Diacylglycerols are recognized by a well conserved protein motif, the C1 domain. It was observed for the first time in protein kinases C but is present also in many other enzyme proteins. Furthermore, diacylglycerols are able to modulate the biophysical properties of biomembranes (Gomez- Fernandez JC et al., Chem Phys Lipids 2007, 148, 1).
A mathematical method of correlating the values of melting points of diacylglycerols with the values of the melting points of the two fatty acids has been described (Maruzeni S, Eur J Lipid Sci Technol 2010, 112, 259).
In bacteria, diacylglycerol is found as a component of the Braun’s lipoprotein which is linked to a large peptidoglycan (murein lipoprotein) and forms the cell envelope of Gram-negative and positive bacteria (review in Hayashi S et al., J Bioenerg Biomemb 1990, 22, 451). The murein lipoprotein of Escherichia coli was discovered by Braun V et al. (Eur J Biochem 1969, 10, 426). They elucidate the structure of the novel lipo-amino acid at the amino terminus of the protein as N-acyl diglyceride-cysteine, i.e., glycerylcysteine containing two ester-linked fatty acids and one amide-linked fatty acid (Hantke K et al., Eur J Biochem 1973, 34, 284). The ester-linked fatty acids are similar to those present in bacteria phospholipids, mainly palmitic acid and cyclopropane fatty acids. The biosynthesis of Braun’s lipoprotein begins with the addition of glycerol, from phosphatidylglycerol, to a cystein residue of a prolipoprotein, followed by the acylation of that glycerol. A cleavage by a peptidase generates a free-form lipoprotein (58 amino acids in E. coli) which will be finally linked to a peptidoglycan.
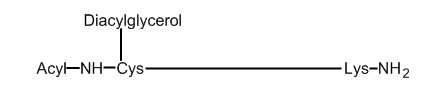
Mature Braun’s lipoprotein
It has been shown that Braun’s lipoprotein was a fully active endotoxic agonist for endothelial cells (Neilsen PO et al., J Immunol 2001, 167, 5231).
Diacylglycerols, which are present at low levels in edible oils (1-6 %), have been used by the food industry as emulsifiers for some time. However, the Kao Corporation introduced a diacylglycerol cooking oil in 1999 that contains more than 80% diacylglycerol. The diacylglycerol in these oils have fatty acids mainly in the 1,3 configuration and are the main components of new cooking oils (ex: Econa, Kao corp., Enova). These oils are proposed to slow the increase of postprandial blood triglycerides to help prevent the accumulation of body fat and high blood cholesterol levels by increasing energy expenditure (review in Yanai H et al., Nutr J 2007, 6:43). In 2000, the FDA granted Kao generally recognized as safe (GRAS) status for their product. Enova has been also ruled safe for human consumption by the Commission of the European Communities in 2006. In the United States, Enova oil is commercially available and may be used in home cooking oil and vegetable oil spreads. Studies indicate that body weight and body fat losses may occur when consuming 10 to 25 grams of Enova oil per day in place of conventional oil. A review of the nutritional characteristics of diacylglycerol oil has been released by Flickinger BD (Flickinger BD et al., Lipids 2003, 38, 129).
A diacylglycerol with the structure of 1,3-di-(w-acetoxyacyl)glycerol has been isolated from the seeds of Xylocarpus granatum, a marine mangrove plant used as a folk medicine in Southern Asia (Huo C et al., Chem Nat Compounds 2013, 48, 934).
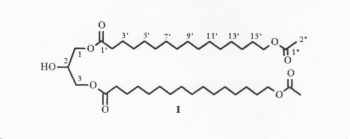
1,3-Di-(w-acetoxyacyl)glycerol
It is well known since 1978 that when foodstuffs are heated, incorporated fats generate glycerol, various acylglycerols and with chloride ions they form mainly 3-chloropropane-1,2-diol esters (Svejkovska B et al., Czech J Food Sci 2004, 22, 190). These compounds are similar to diacylglycerols but with a chlorine atom at the carbon-3 position.
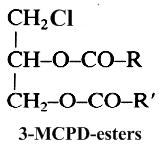
Studies have shown that free 3-chloropropane-1,2-diol (3-MCPD or chlorinated glycerol) is present at only very low levels in most foodstuffs (10-80 mg/kg), while the major part is ester-linked with two fatty acids (up to 6 mg/kg). In refined fats and oils, concentrations of these contaminants can be higher (up to 20 mg/kg) since they are formed during the deodorization step. Based on several toxicological studies, the European Commission has adopted a regulatory limit of 20 m/kg for 3-MCPD in some foodstuffs. At present, no information are given on the toxicity of the fatty esters (Weisshaar R, Eur J Lipid Sci Technol 2008, 110, 671).
Cyclic glycerol esters (tethered lipids) have been isolated from the fruits of the Mediterranean plant Thapsia garganica (Apiaceae) (Liu H et al., J Nat Prod 2004, 67,1439). These diacylglycerols have two of the oxygens of glycerol included in a macrocyclic ring "macrocyclic lipids", the cyclic chain having 18 carbon atoms.
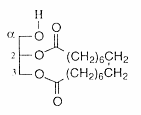
These tethered lipids are unprecedented in the plant kingdom.
Several terpenoic diacylglycerols have been isolated from nudibranch mollusks. Thus, in Archidoris montereyensis, diacylglycerols with the structure of the terpenoic monoglycerides, also described in these animals, but with acetylation of one of the free hydroxyl groups of the glycerol molecule (Gustafson K et al., Tetrahedron 1985, 41, 1101) . Similar acetylated compounds, but with the double bond confined in the B-ring between the carbon 5 and 6, were isolated in Archidoris verrucosa (Cimino G et al., Tetrahedron 1988, 44, 2301). These compounds, which were named verrucosin A and B, may contribute to the survival of the nudibranch in predator-rich areas as they are highly ichthyotoxic, others having feeding-deterrent properties.
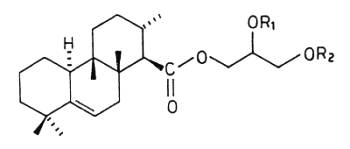
Verrucosin A (R1=H, R2=COCH3) and B (R1=COCH3, R2=H)
An unusual diacylglycerol, archidorin, has been isolated from the mantle of Archidoris tuberculata (Cimino G et al., J Nat Prod 1993, 56, 1642). Glycerol is esterified with tiglic acid (2-methylbut-2-enoic acid) and a diterpenoid clerodane acid at positions sn-1 and sn-2, respectively. Further studies on the Antarctic nudibranch (Austrodoris kerguelenensis) have characterized similar structures but with a diterpenoid moiety (of the clerodane or halimane type) at C-2 of glycerol and linked to an acetyl group at C-1 or C-3 (Gavagnin M et al., Tetrahedron 2003, 59, 5579).
In vegetals, phenolic diacylglycerols have been described. Thus, 1,3-diferulylglycerols have been described in the fruits of Aegilops, a Gramineae which is considered as the ancestors of the cultivated wheat (Cooper R et al., Phytochemistry 1978, 17, 1673). In one form, the two ferulic groups are methylated (see figure below), in the other, only one ferulic group is methylated.
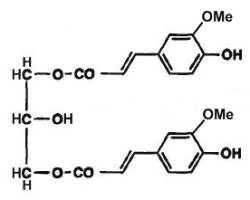
1,3-Diferulylglycerol
That compound and other phenolic glycerides have been isolated from bulbs of Lilium auratum (Shimomura H et al., Phytochemistry 1987, 26, 844). These compounds were identified as 1,2-diferuloylglycerol, 1-feruloyl-2-coumaroylglycerol, 1-coumaroyl-2-feruloylglycerol. These diglycerides have an original structure, with the aromatic acid moieties located at positions C-1 and C-2.
Ferulyl monooleine, a useful sunscreen ingredient, has been produced by lipase-catalyzed transesterification of ethyl ferulate with trioleine (Compton DL et al., JAOCS 2000, 77, 513).
While Eubacteria, plants and animals contain largely diacylglycerol-derived lipids, unusual dialkylglycerols are found in the membranes of many thermophilic bacteria. Specific archaeal tetraethers have also been reported throughout the oceans. These compounds, referred as glycerol dialkyl glycerol tetraethers, have been useful tracers of archaeal biomass. They are reported with little alteration to marine sediments and they have been used to develop proxies for reconstructing sea surface temperature (Schouten S et al., Earth Planet Sci Lett 2002, 204, 265). Tetraeher lipids have been first identified in the subgroup of Thaumarchaeaota (formerly called Crenarchaeota), but they are also present in the other subgroup of Euryarchaeota(Lincoln SA et al., PNAS 2014, 111, 9858).
Non-isoprenoid dialkylglycerol tetraether have been detected in peats but their biological origin is as yet unknown (JS Sinninge Damste et al., Chem Commun 2000, 1683). Similar structures have been described in the genus Thermotoga, thermophilic bacteria of the order Thermotogales (Sinninghe Damste JS et al., Arch Microbiol 2007, 188, 629). These bacteria are commonly found in solfatara springs and in deep-sea marine hydrothermal systems. These lipids consist of two glycerol units connected by two (C32) dimethyl chains derived from diabolic acid (15,16-dimethyltriacontanedioic acid), this acid being also present in these bacteria.
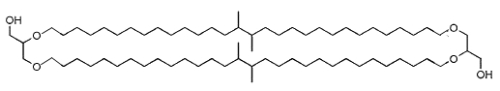
Dialkylglycerol tetraether
Other parent compounds are also present in Thermotogales, they have one to three ester bonds per chain instead of ether bonds. A higher degree of methylation was observed from temperate samples than for the tropical ones (Huguet A et al., Org Geochem 2010, 41, 291). As these bacteria appeared early during the evolution of life on Earth, it may be hypothesized that ether and ester glycerol membrane lipids developed relatively early during evolution.
It must be emphasized that the stereochemistry of the glycerol backbone is 1,2-di-O-alkyl-sn-glycerol.
More branched lipids (isoprenoid lipids) have been described in Archaea (previously Archaebacteria), organisms living in extremely halophilic conditions or in hot and acid springs in deep oceanic waters (Kates M Prog Chem Fats Lipids 1978, 15, 301; Kates M et al., The biochemistry of archaea, Elsevier 1993). These lipids consist of derivatives of a C20,C20-isopranyl glycerol diether (diphytanylglycerol diether) and its dimer (dibiphytanyldiglycerol tetraether). The O-alkyl chains have a branched structure deriving from phytanic acid. In contrast with the structures observed in bacteria, the stereochemistry of the glycerol backbone in Archaea is 2,3-di-O-alkyl-sn-glycerol.
The simplest structure (diphytanylglycerol diether) is now called archaeol. This molecule structure, discovered in extremely halophilic bacteria (Kates M, Prog Chem Fats other Lipids 1978, 15, 301), is found either free or in phospholipid-bound forms. Archaeal lipids have been shown to be biomarker for estimating salinity, especially in hypersaline conditions (Turich C et al., Org Geochem 2011, 42, 1147).
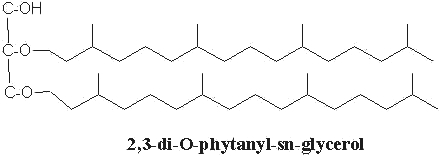
The more complex tetraether structures (dibiphytanyldiglycerol tetraethers) known as caldarchaeol, have been found in membranes from thermoacidophiles and methanogenic bacteria (Langworthy TA, Biochim Biophys Acta 1977, 487, 37).
Investigation of archaeal lipids in suspended particulate matter and surface sediments collected from coastal hypersaline, marine, estuarine and fresh water revealed that the ratio of archaeol to caldarchaeol (ACE index) correlated well with salinity in modern settings, especially hypersaline environments, and the estimated paleosalinity was also consistent with expected absolute salinity for two ancient sediment cores (Turich C et al., Geochim Cosmochim Acta 2007, 71, 3272). Thus, the ACE index can provide paleo-salinity information in the context of paleotemperature as well as soil organic matter input for a given paleo-environment (Wang H et al., Org Geochem 2013, 54, 69).
This structure of caldarchaeol may also contain one to four cyclopentane rings in each of the C40 biphytanyl groups. These tetraethers (crenarchaeols) are present in marine crenarchaeota (now thaumarchaeota), an ubiquitous and abundant component of plankton.
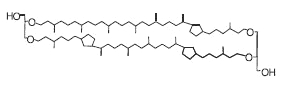
Crenarchaeol
It has been shown that the number of cyclopentane rings is linearly correlated to the seawater temperature (Schouten S et al., Earth Planet Sci Lett 2002, 204, 265). That connection has been also established for lake waters (Powers L et al., Org Geochem 2010, 41, 404). Similarly, experiments using marine species have demonstrated that water temperature is the major factor controlling their lipid membrane distribution. Thus, the relative abundance of the methyl branches correlated with mean annual air temperature and, to a lesser extent, with the pH of the soil (Weijers JWH et al., Geochim Cosmochim Acta 2007, 71, 703). TEX86 (tetraether index of tetrathers consisting of 86 carbons) has been widely used to reconstruct sea surface temperature (Liu ZH et al., Science 2009, 323, 1187).
Another class of caldarchaeols with a hydroxylation in one of the alkyl chains has been described in marine sediments (Huguet C et al., Org Geochem 2013, 57, 107). It was reported that these hydroxylated compounds are widespread and abundant in mesophilic marine and lacustrine environments. Moreover, increasing amounts of these lipids have been reported at higher latitudes and lower water temperatures.
The analysis, occurrence and recognition of sources of glycerol dialkyl glycerol tetraether lipids, their applications as biomarker lipids, and the development and application of proxies based on their distributions have been reviewed (Schouten S et al., Org Geochem 2013, 54, 19).
A review of the distribution of glycerol ether lipids in several species of Archaea, including six species belonging to four different families, has been done in order to reassess potential sources of archaeal lipids in marine, lacustrine and terrestrial environments (Bauersachs T. et al., Org Geochem 2015, 83-84, 101-8).
A new series of archaeal lipids has been discovered in marine sediments but also in cultured methanogenic Archaea (Methanothermococcus) (Liu X.L. et al., Org Geochem 2012, 43, 50). These lipids (diphytanylglycerol diether) are not very different from caldachaeol but with one glycerol unit missing and with each biphytanyl moiety possessing a terminal OH group.
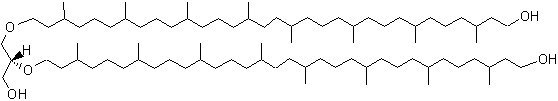
Diphytanylglycerol diether
Some of these molecules have up to five cyclopentane rings in the isoprenoid chains. Diagenesis of caldarchaeol is a possible source of these new compounds in sediments but a biological origin should not be excluded.
It is noticeable that extreme halophiles contain only archaeol-derived lipids, the methanogens bacteria contain both archaeol- and caldarchaeol-derived lipids, and the thermoacidophiles contain largely caldarchaeol-derived lipids.
Unsaturated terpenoid chains were found in Thermococcus hydrothermalis, an Archaea living in deep-sea hydrothermal vents (Lattuati A et al., Lipids 1998, 33, 319).
These lipids have been discovered in peat bogs and soils and a survey of their distribution in various locations has shown that they can be used in paleo-environmental studies to estimate annual mean air temperature and soil pH (Weijers J et al., Geochem Cosmochim Acta 2007, 71, 703).
Since the discovery of archaeal lipids, several research laboratories have focused specifically their efforts on the structural and physicochemical characterizations of tetraether-type lipids as well as on their biotechnological applications (Hanford MJ et al., Appl Biochem Biotechnol 2002, 97, 45). These synthetic lipids could represent an elective material for the construction of a new generation of stable liposomes (for drug or gene delivery) and artificial membranes of technological interest (review in: Jacquemet A et al., Biochimie 2009, 91, 711). They have been also evaluated for their use in the lubrication of engines. This research has resulted in a patent for novel boundary lubricants (Chang, E. L. 1992, US patent, no. 5,098,588).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire