
Sterols may be found either as free sterols, acylated (sterol esters), alkylated (steryl alkyl ethers), sulfated (sterol sulfate), or linked to a glycoside moiety (steryl glycosides) which can be itself acylated (acylated sterol glycosides).
Sterol biosynthesis is nearly ubiquitous among eukaryotes, it is almost completely absent in prokaryotes. They are not found in Archaea and the proven occurrences in bacteria are sparsely distributed and yield a limited array of products. The proteobacterium Methylococcus capsulatus and the planctomycete Gemmata obscuriglobus (Pearson A et al., PNAS 2003, 100, 15357) and some members of the myxobacteria are proven steroid-producing bacteria. As a result, the presence of diverse steranes (saturated 4-cycle skeleton) in ancient rocks is used as evidence for eukaryotic evolution 2.7 billion years ago.
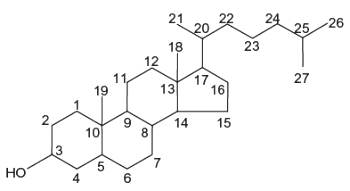
Structure of sterols with carbon numbers
Sterols are derived from the same squalene precursor as hopanoids but, in marked contrast, they are known to have an oxygen-dependent biosynthesis beginning with the formation of the first intermediate, 2,3- oxidosqualene. There is a close connection between modern-day biosynthesis of particular triterpenoid biomarkers and presence of molecular oxygen in the environment. Thus, the detection of steroid and triterpenoid hydrocarbons far back in Earth history has been used to infer the antiquity of oxygenic photosynthesis (Summons RE et al., Phil Trans R Soc B 2006, 361, 951). It has been hypothesized that increased levels of O2 in the atmosphere not only made the evolution of sterols possible, but that these sterols may in turn have facilitated the birth of complex organisms (the eukaryotes) (Chen LL et al., Biochem Biophys Res Comm 2007, 363, 885), likely in providing them with an early defense mechanism against O2 (Galea A et al., Free Rad Biol Med 2009, 47, 880; Brown AJ et al., Evolution 2010, April 14).
FREE STEROLS
Sterols form an important group among the steroids. Unsaturated steroids with most of the skeleton of cholestane containing a 3b-hydroxyl group and an aliphatic side chain of 8 or more carbon atoms attached to position 17 form the group of sterols.
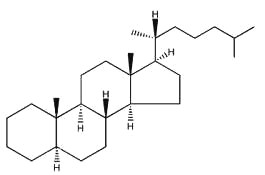
5a-cholestane
They are lipids resistant to saponification and are found in an appreciable quantity in all animal and vegetal tissues. Furthermore, cholestane may be considered as a biological marker compound valuable in the assessment of marine sediment maturity, even after hundreds of millions of years (Mackenzie AS et al., Science 1982, 217, 491). Sterols may include one or more of a variety of molecules belonging to 3-hydroxysteroids, they are C27-C30 crystalline alcohols (in Greek, stereos, solid). These lipids can be classed also as triterpenes, as they derive from squalene which gives directly by cyclization, unsaturation and 3b-hydroxylation, lanosterol in animals or cycloartenol in plants.
In the tissues of vertebrates, the main sterol is the C27 alcohol cholesterol (Greek, chole, bile), particularly abundant in adrenals (10%, w/w), nervous tissues (2%,w/w), liver (0.2%,w/w) and gall stones. The vertebrate brain is the most cholesterol-rich organ, containing roughly 25% of the total free cholesterol present in the whole body. Its fundamental carbon structure is a cyclopentanoperhydrophenanthrene ring (also called sterane). It was the first isolated sterol around 1758 by F.P. Poulletier de La Salle from gall stones. In 1815, it was isolated from the unsaponifiable fraction of animal fats by M.E. Chevreul who named it cholesterine (Greek, khole, bile and stereos, solid). The correct formula (C27H46O) was proposed in 1888 by F. Reinitzer but structural studies from 1900 to 1932, mainly by H.O. Wieland "on the constitution of the bile acids and related substances" (Nobel Prize Chemistry 1927) and by A.O.R. Windaus on "the constitution of sterols and their connection with the vitamins" (Nobel Prize Chemistry 1928), led to the exact steric representation of cholesterol. In 1936, Callow RK and Young FG have designated steroids all compounds chemically related to cholesterol. The main steps of cholesterol research have been reviewed up to the year 2000 (Vance DE et al., Biochim Biophys Acta 2000, 1529, 1). It must be noticed that the central role of cholesterol in atherogenesis has been proposed by a young Russian pathologist in Saint Petersburg in 1913 (Steinberg D, J Lipid Res 2013, 54, 2946).
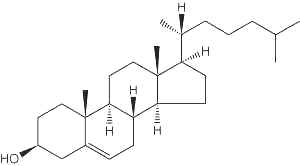
Cholesterol
Cholesterol is found in high concentrations in animal cell membranes, typical concentrations (expressed as molar percentage of total lipids) being about 30 mol%, ranging up to 50mol% in red blood cells and as high as 80 mol% in the ocular lens membranes (Li LK et al., J Lipid Res 1985, 26, 600). Consequently, cholesterol has numerous functions in membranes ranging from metabolism, as a precursor to hormones and vitamins, to providing mechanical strength and a control of the phase behavior of membranes (Rog T et al., Biochim Biophys Acta 2009, 1788, 97). It became clear that the key role of cholesterol in the lateral organization of membranes and its free volume distribution seems to be involved in controlling membrane protein activity and "raft" formation (review in Barenholz Y, Prog Lipid Res 2002, 41, 1). At the cellular level, cholesterol may be replaced to some extent by some other sterols with minor modifications of the side chain (campesterol, b-sitosterol) (Xu F et al., PNAS 2005, 102, 14551). Cholesterol is abundant in the femoral gland of the male lizard Acanthodactylus boskianus which uses it as a scent marking pheromone to establish dominance hierarchies (Khannoon ER et al., Chemoecology 2011, 21, 143).
In addition to these roles, cholesterol can form ester linkages with a class of secreted polypeptide signaling molecules encoded by the hedgehog gene family. These proteins function in several patterning events during metazoan development (Mann R et al., Biochim Biophys Acta 2000, 1529, 188).
Sponges, a primitive group of multicellular organisms (Poriphera), represent the richest source of bizarre sterols found in nature. Most sponges have the general sterol structure found in animals, plants, and fungi., i.e. cholesterol and sterols, but bearing one to three extra carbon atoms at C24. These side chains have been isolated with such unusual features as quaternary alkyl groups, cyclopropane and cyclopropene rings, allenes, and even acetylenes (Giner JL, Chem Rev 1993, 93, 1735).
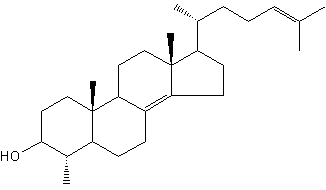
24-Isopropylcholesterol is abundant and characteristic (with its analogue unsaturated at C22-C23) of the class Demospongiae. This sterol is absent in "true animals", the eumetazoans (cnidarians and bilaterian animals).
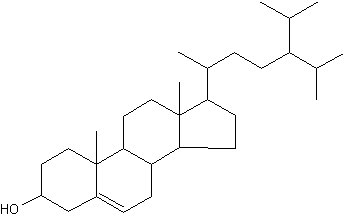
24-Isopropylcholesterol
This demosponge sterane is abundant in sediment dating from the Neoproterozoic era (1,000-542 million years) and is the oldest evidence for animals in the fossil record (Love GD et al., Nature 2009, 457, 718).
Among the large list of sterols with cyclopropane ring, Nicasterol was identified in a Demospongiae, Calyx nicaensis.
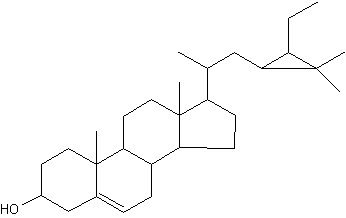
Nicasterol
While cholesterol was considered to be nearly absent in vegetal organisms, its presence is now largely accepted in higher plants. It can be detected in vegetal oils in a small proportion (up to 5% of the total sterols) but remains frequently present in trace amounts. An unusual relatively high content of cholesterol was described in camelina oil (about 200 mg per kg) (Shukla VKS et al., JAOCS 2002, 79, 965). However, several studies have revealed the existence of cholesterol as a major component sterol in chloroplasts, shoots and pollens. Furthermore, cholesterol has been detected as one of the major sterols in the surface lipids of higher plant leaves (rape) where he may amount to about 72% of the total sterols in that fraction (Noda M et al., Lipids 1988, 23, 439). Cholesterol is also dominant in most all Rhodophyceae algae, it is the only sterol presesnt in Laurencia paniculata (Al Easa H et al., Phytochemistry 1995, 39, 373).
|
|
|
|
In late-step synthesis of cholesterol, discrete oxidoreductive and/or demethylation reactions occur, which start with the common precursor lanosterol. Lanosterol is also found as a major constituent of the unsaponifiable portion of wool fat (lanoline) : about 15%. It has been shown that the bacterium (planctomycete), Gemmata obscuriglobus, is able to synthesized lanosterol and its uncommon isomer, parkeol (Pearson A et al., PNAS 2003, 100, 15357). No subsequent modifications of these sterols were observed.
Several lanosterol derivatives have been identified in methanotrophic bacteria. The most abondant derivative, 4-methylcholestan-8(14),24-dien-3b-ol, has been found first in Methylococcus capsulatus (Bouvier P et al., Biochem J 1976, 159, 267) and later in other similar bacteria.
4-Methylcholestan-8(14),24-dien-3b-ol
Several compounds with the lanostane nucleus (ganoderates, lucidenates) have been isolated from a mushroom Polyporaceae (Ganoderma lucidum) (Boh B et al., Biotechnol Ann Rev 2007, 13, 265). These sterols could be related to the use of that mushroom in the traditional Chinese and Japanese medicine to cure several pathologies (hypertension, hypercholesterolemia, hepatitis, gastritis, diabetes, bronchitis, and cardiovascular problems) (Lee I et al., J Nat Prod 2010, 73, 172). One of several compounds named ganoderic acid A is shown below.
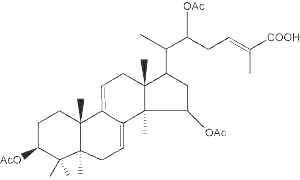
One of the ganoderic acids from Ganoderma lucidum
Animal tissues contain in addition to cholesterol small amounts of 7-dehydrocholesterol which, on UV irradiation, is converted to vitamin D3 (cholecalciferol).
Desmosterol (24-dehydrocholesterol), an intermediate between lanosterol and cholesterol, has been implicated with myelination processes. While high desmosterol levels could be detected in the brain of young animals (Paoletti R et al., J Am Oil Chem Soc 1965, 42, 400) no desmosterol was found in the brain of adult animals. It is also known as an abundant membrane component in some mammalian cells, such as spermatozoa and astrocytes (Lin DS et al., J Lipid Res 1993, 34, 491 – Mutka AL et al., J Biol Chem 2004, 279, 48654). Inability to convert desmosterol to cholesterol leads to the human disorder desmosterolosis (a severe developmental defect and cognitive impairment) (Waterham HR et al., Am J Hum Genet 2001, 69, 685). Desmosterol and 22-dehydrocholesterol are present in high concentrations in red algae.
In microalgae, sterols usually possess C27C29 skeletons, with differences in alkylation at C-24 and double bonds in the nucleus (D5, D8, D8(14)) and side chain (D22, D24, D24(28). Dinoflagellates are unusual in terms of steroid composition, as they often contain sterols with additional methyl groups C-4 and C-23.
Gorgosterol was discovered by Bergmann in 1943 (Bergmann W et al., J Org Chem 1943, 8, 271) and named after the coral like animals from which it was isolated. It was later found that gorgosterol is actually produced by zooxanthellae (intracellular photosynthetic dinoflagellate symbionts). The original structure of gorgosterol, which bears an unusual C-23 methyl group and a cyclopropane ring in the side chain contributed greatly to the renewed interest in marine sterols.
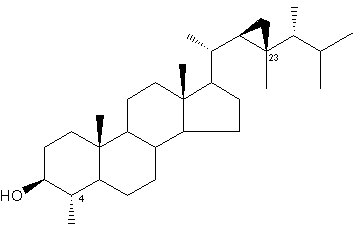
Gorgosterol
For example, dinosterol (4a,23,24-trimethyl-5a-cholest- 22E-en-3b-ol) is typically found in dinoflagellates where it is generally accepted as a reliable biomarker (Boon JJ et al., Nature 1979, 277, 125).
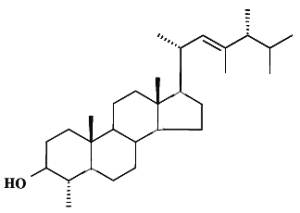
Dinosterol
23-Methyl sterols have been also reported in diatoms and their sterane equivalents were also unambiguously identified in sediments and petroleum from the late Jurassic onwards (Rampen S et al., Org Geochem 2009, 40, 219).
Oxysterol : An important oxysterol, 24S-Hydroxycholesterol, is an enzymatically oxidized product of cholesterol mainly synthesized in the brain. It was detected in 1953 in horse brain and named "cerebrosterol" (Ercoli A et al., J Am Chem Soc 1953, 75, 3284). It was proposed that this oxysterol could be a biochemical marker for Alzheimer’s disease (Lütjohann D et al., J Lipid Res 2000, 41, 195). Furthermore, 24S-Hydroxycholesterol is reported to be protective against b-amyloid peptide, the amyloidogenic peptide found in plaques in Alzheimer disease brain (Brown J et al., J Biol Chem 2004, 279, 34674).
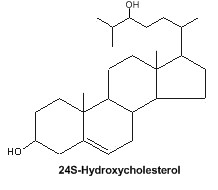
It has been shown that fluctuations in the levels of cholesterol oxidation products may correlate with the onset of Alzheimer’s disease and Parkinson disease (review in : Marwarha G et al., Exp Gerontol 2015, 68, 13).
Delta(7)-dafachronic acid, or (5a)-3-oxocholest-7-ene, a member of the class of dafachronic acids, is a cholestane derivative in which the methyl group at position 26 has been oxidised to the corresponding carboxylic acid. It could play an important role in the diet-mediated longevity enhancement.
A review of the other oxysterols is given elsewhere. Various aspects of oxysterols biology, mainly in bile acid metabollism, has been reviewed (Crosignani A et al., Clin Chim Acta 2011, 412, 2037).
Starfishes contain a great number of polar steroids (oxysterols) characterized by numerous hydroxylations which have no counterpart in the animal kingdom. As an example, the structure of a 5a-cholestane-hexaol present in a Far Eastern starfish, Henricia leviuscula, is given below (Ivanchina NV et al., J Nat Prod 2006, 69, 224).
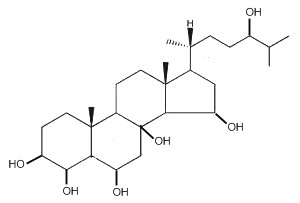
5a-Cholestane-hexaol
Chlorinated cholesterol : The myeloperoxidaseH2O2Cl system can react with the double bond in the 2nd ring of cholesterol to generate a-chlorohydrins (6-b-chloro-cholestane-( 3b,5a)-diol) and other chlorinated products (Heinecke JW et al., Biochemistry 1994, 33, 10127). Because chlorohydrins are quite stable, chlorinated sterols may prove useful as markers for lipoproteins oxidatively damaged by activated phagocytes which are known to secrete myeloperoxidase.
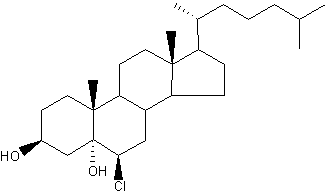
Cholesterol a-chlorohydrins
These products were also formed in LDL (Hazen SL et al., 1996) and cell membranes following exposure to HOCl or the myeloperoxidase system (Carr AC et al., Arch Biochem Biophys 1996, 332, 63). In cells, the formation of cholesterol chlorohydrins could be potentially disruptive to cell membranes as it results in cell lysis and death. They could also be potential biomarkers for oxidative damage associated with neutrophil/monocyte activation.
Phytosterols : In higher plants, the first sterols were isolated by Hesse O (1878) from the Calabar beans (Phytostigma venenosum) which coined the term "phytosterine". This substance was later named stigmasterol (Windaus and Hault, 1906) from the plant genus. The denomination "phytosterol" was proposed in 1897 (Thoms H) for all sterols of vegetal origin. Chemically, these sterols have the same basic structure as cholesterol but differences arise from the lateral chain which is modified by the addition of one or two supernumerary carbon atoms at C-24 with either a or b chirality. The 24-alkyl group is characteristic of all phytosterols and is preserved during subsequent steroid metabolism in both fungi and plants to give hormones that regulate growth and reproduction in a manner similar to animals.
The study of the physical properties of model membranes mimicking plant plasma membranes suggests that phytosterols are more efficient than cholesterol in extending the temperature range in which membrane-associated biological processes can take place (Beck JG et al., FASEB J 2007, 21, 1714). These conclusions are in accordance with the fact that plants have to face higher temperature variations than animals.
Most phytosterols are compounds having 28 to 30 carbon atoms and one or two carbon-carbon double bonds, typically one in the sterol nucleus and sometimes a second in the alkyl side chain.
All phytosterols were shown to derive in plants from cycloartenol and in fungi (including yeasts), as in vertebrates, from lanosterol, both direct products of the cyclization of squalene.
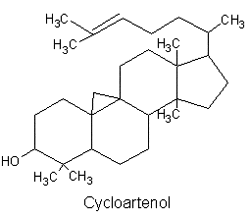
There is direct evidence to indicate that the biosynthetic pathway for phytosterol via lanosterol exists also in plant cells. This new biosynthetic pathway was designated the lanosterol pathway" (Ohyama K et al., PNAS 2009, 106, 725).
More than 250 different types of phytosterols have been reported in plant species. Representatives of these sterols are campesterol, b-sitosterol and stigmasterol (in soybean oil). b-Sitosterol is present in all plant lipids and is used for steroid synthesis. Stigmasterol, which is used for the synthesis of progesterone and vitamin D3, is known as "Wulzen factor", a potential anti-inflammatory compound. Its action is mediated by the inhibition of several pro-inflammatory and matrix degradation mediators involved in osteoarthritis-induced cartilage degradation (Gabay O et al., Osteoathr Cartil 2010, 18, 106).
They all belong to the group of 4-desmethyl sterols and account for 30%, 3%, and 65%, respectively, of human dietary phytosterol intake. In brown algae (Phaeophyceae) the dominant sterol is fucosterol and cholesterol is present only in low amounts. Fucosterol (24-ethylidene cholesterol) is a sterol present in algae, seaweed and diatoms. It exhibits various biological therapeutics, including anticancer, antidiabetic, antioxidant, hepatoprotective, antihyperlipidemic, antifungal, antihistaminic, anticholinergic, antiadipogenic, antiphotodamaging, anti-osteoporotic, blood cholesterol reducing, blood vessel thrombosis preventive and butyrylcholinesterase inhibitory activities (Abdul Q.A. et al., J Sci Food Agric 2016, 96, 1856).
An important sterol from yeast and ergot is the C28 compound ergosterol (mycosterol). Following the discovery of ergosterol by Tanret C (Compt rend Acad Sci Paris, 1889, 108, 98), Gerard E (Compt rend Acad Sci France 1892, 114, 1544; idem 1898, 126, 909) observed that not only ergot, but fungi in general, contain it. Gerard was the first to recognize ergosterol in yeast. Upon irradiation, this sterol gives rise to vitamin D2 (calciferol) which, once absorbed, does not affect vitamin D status (Stephensen CB et al., J Nutr 2012, 142, 1246).
As ergosterol is a cell membrane component largely restricted to fungi, its amount in environmental matrices may be used as an index molecule for these micro-organisms in a living biomass (Barajas-Aceves M et al., J Microbiol Methods 2002, 50, 227; Charcosset JY et al., Appl Environ Microbiol 2001, 67, 2051).
|
|
|
|
|
|
|
|
||
An isomeric form of ergosterol, antrosterol (ergosta-7,9,22-trien-3b-ol), from the fungus Antrodia camphorata, is efficient in the protection from liver damage through its anti-inflammation capacity (Huang GJ et al., Food Chem 2012, 132, 709). This compound is likely at the origin of the use of the fungus as a traditional Chinese medicine for the treatment of drug intoxication, diarrhoea, hypertension and cancer.
Considerable variability in the concentration of free sterols was observed among different oils. While concentrations lower than 100 mg/100 g are found in oils from coconut, palm, olive, and avocado, concentrations between 100 and 200 mg/100 g are found in oils from peanut, safflower, soybean, borage, cottonseed, and sunflower, and concentrations between 200 and 400 mg/100 g are found in oils from sesame, canola, rapeseed, corn, and evening primrose (Phillips KM et al., J Food Comp Anal 2002, 15, 123). A unique online database (EuroFIR-BASIS) contains information on the content of 18 individual phytosterols in 91 different plants and plant based foods.
Phytosterols account for a substantial portion of total dietary sterols in vertebrates but they are excluded from the body. Accumulation of other sterol than cholesterol is prevented at the level of the intestinal epithelium concurrently with a facilitation of biliary excretion of phytosterols. Phytosterols produce a wide spectrum of biological activities in animals and humans. They are considered efficient cholesterol-lowering agents (Trautwein EA et al., Eur J Lipid Sci Technol 2003, 105, 171; Ostlund RE, Lipids 2007, 42, 41). In addition, they produce a wide spectrum of therapeutic effects including anti-tumor properties. Further data on their metabolism and potential therapeutic action can be found in a review article (Ling WH et al., Life Sci 1995, 57, 195). A review of physiologic and metabolic aspects related to these cholesterol-lowering properties may be consulted (Brufau G et al., Nutr Res 2008, 28, 217). The interest of adding sterols and stanols to human food to improve health has been discussed (Caswell H et al., Nutr Bull 2008, 33, 368). Clinical experiments have shown that only high amounts of stanols (about 9 g/day) can decrease serum b-carotene concentrations, without altering those of vitamins A, D and E (Gylling H et al., Clin Nutr 2010, 29, 112).
Saringosterol, a derivative of fucosterol, discovered in several brown algae (Phaeophyta), such as Lessonia nigrescens and Sargassum ringgoldianum, has been shown to inhibit the growth of Mycobacterium tuberculosis (Wachter GA et al., J Nat Prod 2001, 64, 1463). This phytosterol compares well with the tuberculosis drug rifampin, without appreciable toxicity against mammalian cells, and may be the source for future tuberculosis drugs.
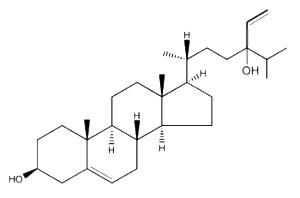
Saringosterol
The European Commission authorized in 2004 the addition of phytosterols and phytostanols in food products with conditions of labeling including their amount per 100 g and the statement that the human consumption of more than 3 g/day should be avoided.
As cholesterol, phytosterols may undergo oxidative processes. These oxyphytosterols have been shown to have beneficial biological properties which deserve further investigations (Hovenkamp E et al., Prog Lipid Res 2008, 47, 37).
Phytostanols are a fully-saturated subgroup of phytosterols (they contain no double bonds). They are in general produced by hydrogenation of phytosterols. These saturated sterols are found in very small amounts in plant products, such as nuts, seeds and legumes but in higher amounts in tissues of a few cereal species. To improve their solubility, plant stanols are often
combined with a fatty acid ester to produce plant stanol esters. The most generally found stanol is sitostanol.
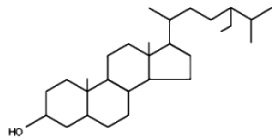
Sitostanol
Stanols often occur in dinoflagellates but are not common in other marine microalgae. Hence, dinoflagellates are often the major direct source of 5a(H)-stanols in marine sediments (Robinson N et al., Nature 1984, 308, 439).
Fully saturated sterols are also found in animals but are of bacterial origin. Thus, the 5b(H)-stanol, coprostanol, constitutes approximately 60% of the total sterols in human faeces.
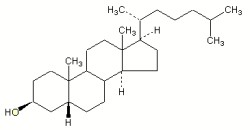
Coprostanol
Although practical, the ancient distinction between zoosterols, mycosterols and phytosterols is no more used, since the same sterol may have different sources, but the appellation phytosterol is actually more frequently used.
Sterols are often isolated in the unsaponifiable fraction of any lipid extract and determined by various chromatographic procedures (HPLC or GLC).
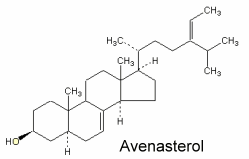
Avenasterol can be isolated from oat oil. This sterol was shown to protect specifically frying oils from oxidation owing to its ethylidene group in the side chain (White PJ et al., JAOCS 1986, 63, 525).
An extensive review on the diversity, analysis, and health-promoting uses of phytosterols and phytostanols may be consulted with interest (Moreau RA et al., Prog Lipid Res 2002, 41, 457).
It must be noticed that sterols are of widespread occurrence and have a long persistency in sediment and, thus, are found in aged geological samples (Nishimura M et al., Geochim Cosmochim Acta 1977, 41, 38).
Coprostanol, dihydrocholesterol and stigmastanol are frequently found in these sediments.
Completely saturated sterols (steranes) are derived from steroids or sterols via diagenetic degradation and saturation. They are sometimes used as biomarkers mainly for the presence of life in sedimentary organic matter and petroleum. The most abundant have 27 to 29 carbon atoms, although C30 dinosterol derivatives also occur. Thus, the most common steroids found in sediments are the regular C27-C29 steranes. Sterenes are key intermediates in the conversion of oxygenated steroids into saturated steranes. A broad range of monounsaturated sterenes have been detected in immature sediments (Lu H et al., Org Geochem 2009, 40, 902).
24-Isopropylcholestane, the hydrocarbon remains of C30 sterols produced by marine demosponges, records the presence of Metazoa in the geological record during the neoproterozoic era (1,000-542 millions years ago) (Love GD et al., Geochem Cosmochim Acta 2006, 70, A625). This sterol represents the oldest evidence for animals in the fossil record and may provide a powerful biomarker for early animal diversification (Kodner RB et al., PNAS 2008, 105, 9897). As far as known, 24-isopropylcholesterol is not synthesized by eumetazoans (cnidarians plus bilaterian animals).
STEROL ESTERS
If sterols occur in the free state in cellular membranes in intimate association with phospholipid molecules, they are frequently found esterified to fatty acids. In animal tissues, especially in the liver, adrenals and plasma lipids (more the 70% in circulating lipoproteins), cholesterol is esterified by a variety of fatty acids and most frequently by essential fatty acids, thus forming cholesterol esters. Thus, the esterification of cholesterol with arachidonic acid gives cholesteryl arachidonate. Sterol esters are important but highly variable components of the yeast cell with values ranging from traces to 50% of the total lipids.
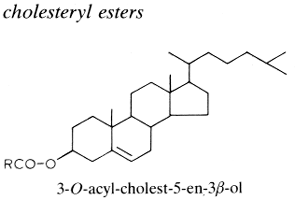
The chemical bonding between the sterol and the fatty acid is hydrolyzed or transesterified much more slowly than most O-acyl lipids. In animals, the esterification of free cholesterol within intestinal cells (by acyl CoA:cholesterol acyltransferase, ACAT) allows the cholesterol to be stored as a neutral lipid in cytosolic droplets and in the packing of cholesterol into lipoprotein particles for export via the plasma to liver cells. The secretions of human meibomian glands (sebaceous glands at the rim of eyelids) are particularly rich in cholesterol esters (about 30% of the lipid pool) which are characterized by a saturated or monounsaturated fatty acid moiety with C18-C34 carbon chain (Butovich IA, J Lipid Res 2009, 50, 501). Cholesteryl linoleate and cholesteryl arachidonate present in nasal fluid have been shown to contribute to the inherent antibacterial activity of that secretion (Do TQ et al., J Immunol 2008, 181, 4177). Curiously, it has been shown that the cholesteryl esters isolated from squid oil contain only saturated fatty acids (Galliani G et al., Eur J Lipid Sci Technol 2016, 118, 453).
Cholesteryl nitrolinoleate, a nitrated lipid has been detected in human blood plasma and lipoproteins (Lima ES et al., J. Lipid Res 2003, 44, 1660). Nitrated cholesterol linoleate can be considered as potential indicator of the chain-breaking antioxidant role of NO during lipid peroxidation, as previously reported (Rubbo, H et al., Arch Biochem Biophys 1995, 324, 15). Nitrated linoleic acid has been well studied in several biological models and appears now as a potent signaling molecule. The level of cholesteryl nitrolinoleate was shown to be largely increased after macrophage activation, suggesting that lipid nitration occurs as part of the response to inflammatory stimuli (Ferreira AM et al., Biochem J 2009, 417, 223). That bioactive lipid may act as a down-regulator of inflammatory responses.
In plants, several sterol esters can be found in cell membranes and seed oils, such as ergosteryl, stigmasteryl and b-sitosteryl esters. In bryophytes (Hepaticae), cycloartenol and stigmasterol esters have been isolated (Toyota M et al., J Oleo Sci 2006, 55, 579). The relative importance of esterified sterols depends on the vegetal oil, 50-70% being found in oils from evening primrose, avocado, rapeseed, canola, corn, peanut, and sunflower, 30-50% in oils from borage, olive, sesame, coconut, and cottonseed, and less than 30% in oils from safflower, palm, and soybean. Thus, a large variation in the content and distribution of the sterol fractions between different vegetal oils can be observed (Verleyen T et al., JAOCS 2002, 79, 117). Variability reflects also differences in processing of oils and in growing season of the plant source (Phillips KM et al., J Food Comp Anal 2002, 15, 123).
In addition to variations in quantities, yeast sterol esters have been found to vary in both the sterol and fatty acid components. Fatty acids have a carbon chain from 12 up to 18 carbon atoms, saturated or having one to three double bonds. Investigations have shown than more than 20 different sterols occur in the esterified form.
In addition, cholesterol can form ester linkages with a class of secreted polypeptide signaling molecules encoded by the hedgehog gene family. These proteins function in several patterning events during metazoan development (Mann R et al., Biochim Biophys Acta 2000, 1529, 188). Observations suggest that cholesterol modification of polypeptides may be not unique to the Hedgehog proteins.
The life cycle of sterol esters, their synthesis, storage and degradation, has been reviewed (Athenstaedt K et al., Cell Mol Life Sci 2006, 63, 1355).
The presence of these esterified forms justifies a previous saponification if an estimation of the total sterol content is needed.
Steryl alkyl ethers have been reported to occur only in marine sediments up to Cretaceous age. They were first reported in sediments from Walvis Bay (Boon JJ et al., Marine Chem 1979, 7, 117). Mass spectral characteristics indicate that these steryl alkyl ethers consist of C27C29 sterols with 1-2 double bonds, that are ether-bound to C8C9 alkyl chains. The detailed characterization of the structures of some of the dominant sedimentary steryl alkyl ethers have been reported (Schouten S et al., Org Geochem 2005, 36, 1323). Mass chromatography revealed that they are mainly composed of C27C29 steroid moieties with one double bond and ether-bound to a C10-C12 alkyl moiety.
One of the most frequent structure (cholest-5-enyl 3b-(3-dodecanyl) ether) found in Pleistocene Atlantic sediments is shown below.
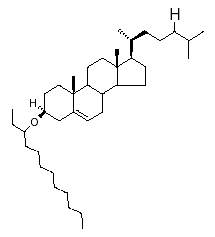
Based on their occurrence in sediments with a high diatom input, it was suggested that yet unknown diatoms should be a direct biological source.
Glycosylated steryl derivatives are synthesized by most plants and fungi, few bacteria and some animal cells.
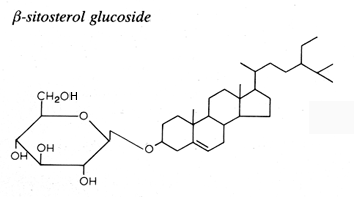
This family consists of one carbohydrate unit linked to the hydroxyl group of one sterol molecule.
The sterol moiety was determined to be composed of various sterols: campesterol, stigmasterol, sitosterol, brassicasterol, dihydrositosterol and cholesterol. The sugar moiety is composed of glucose, xylose and even arabinose (Graminae).
In bacteria, Helicobacter was shown to be particularly rich in cholesterol glucosides (up to 33% of total lipids), thus suggesting that these molecules may be important chemotaxonomic markers for these species (Haque M et al.,J Bacteriol 1995, 177, 5334).
The presence of cholesterol diglucoside was reported in a procaryote (Acholeplasma axanthum) (Mayberry WR et al., Biochim Biophys Acta 1983, 752, 434).
In plants, it has been suggested that sterol glucosides participate to the synthesis of cellulose (Peng L et al., Science 2002, 295, 147). This glycolipid is used as a substrate to produce higher homologues of the cellobioside type with b
eta-1,4-linked glucosyl residues. The resulting disaccharide is split off and used as primer for further elongation to cellulose.
The presence of sterol-glucosides is poorly documented in mammalian cells. Indications of the existence of glucosyl- beta-D-cholesterol or 1-O-cholesteryl-beta-D-glucopyranoside in mammalian cells were first provided by Kunimoto (Kunimoto S. et al., Cell Stress Chaperones 2000, 5, 3). It has been demonstrated that mammalian (mice) tissues contain glucosyl-beta-D-cholesterol formed by transglucosylation through beta-glucosidases using glucosyl ceramide as donor (Marques AR et al., J Lipid Res 2016, 57, 451).
Cholesterol glucuronide was isolated from human liver (Hara A et al., Lipids 1982, 17, 515), its content being about 33 nmol/g wet tissue. The authors have isolated this compound from the acidic lipid fraction and emphasized that it cannot be distinguished readily from ganglioside GM4 by TLC. Cholesterol glucuronide is presumably synthesized in the liver and some of it enters the bloodstream (where it is present at a concentration of about 6 mg/ml), the rest being probably eliminated into the bile..
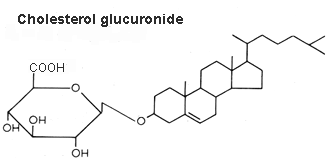
An important review of the structural diversity and occurrence of steryl glycosides, their biosynthesis, their intracellular localization and their functions my be consulted for further details (Grille S et al., Prog Lipid Res 2010, 49, 262).
These compounds are formed when a fatty acid is found acylated at the primary alcohol group of the carbohydrate unit (glucose or galactose, see figure above) in the steryl glycoside molecule (Lepage M, J Lipid Res 1964, 5, 587). Thus, 6′-palmitoyl-b-D-glucoside of b-sitosterol is the major species (51%) detected in potato tubers while 6′-linoleoyl-b-D- glucoside of b-sitosterol is predominant (47%) in soybean extracts. In these products, other fatty acids were also detected (16:1, 18:1, 18:3). More complex molecules were reported in some aquatic plants (Pistia stratiotes) where sitosterol glycosides are acylated with acetyl groups (C2′ and C4′) beside a stearyl residue (C6′) on the sugar (Della Greca M et al. Phytochemistry 1991, 30, 2422).
In a recent survey of 48 plant sources, it was shown that acylated steryl glucoside is present at concentrations from 1 to 125 mg per 100 g fresh weight in all kinds of vegetable parts (fruit, tuber, root, stem, leaf, cereals), the acylated form being 2 to 10 times more abundant that the non acylated sterol glycoside itself (Sugawara T et al., Lipids 1999, 34, 1231).
In a plant (Edgeworthia chrysantha), it was demonstrated the presence of two steryl glycosides (sitosterol glucopyranoside acylated with linoleic or linolenic acid) which have piscicidal activities (at a concentration of 100 ppm they kill Oryzias latipes within about two hours) (Hashimoto T et al. Phytochemistry 1991, 30, 2927).
Several sulfated and polyhydroxylated steroid glycosides have been described in the starfish Linckia laevigata (Kicha AA et al., Chem Nat Compounds 2007, 43, 76). The most unusual chemical structure is that of linckoside, a diglycoside compound, with one glycoside moiety being a xylopyranosyl, the other a methyl xylopyranosyl.
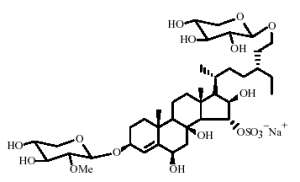
Four other homologue structures but with only one glycoside moiety have been also described.
More than 70 sterol sulfates have been described, mainly in marine invertebrates (Riccio R et al., Chem Rev 1993, 93, 1839). These compounds have one to three sulfate groups, linked to the tetracycle core or to the lateral chain. They have characteristic antibacterial and antiviral properties.
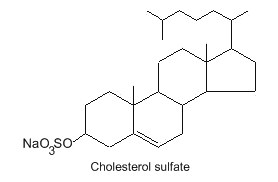
Sulfate ester of cholesterol occurs in mammalian cells. Thus, a cholesterol-3-O-sulfate has been detected in red blood cells and mainly in skin keratinized layers where it plays a role in the desquamation process.
Squalamine, a condensation of cholestane 24-sulfate with spermidine in the 3b position, was discovered in shark stomach and was shown to be an efficient and broad-spectrum antibiotic (Moore KS et al., Proc Natl Acad Sci USA 1993, 90, 1354). This compound which may be a potential host-defense agent cannot be considered as a true lipid since it is water soluble. Later studies have shown that squalamine inhibits angiogenesis and endothelial cell proliferation (Hao D et al., Clin Cancer Res 2003, 9, 2465) and thus could be of value for fighting against several pathologies (cancer, macular degeneration). The presence of squalamine in lamprey white blood cells which are immune cells makes it reasonable to speculate that this molecule evolved in lower vertebrates as an immune effector (Yun SS et al., J Lipid Res 2007, 48, 2579).
A sulfated oxysterol, a cholesterol-3-O-sulfate hydroxylated on C-25, has been found at high levels in the rat hepatocytes (mitochondria, nucleus) (Ren S et al., J Lipid Res 2006, 47, 1081). Later, that sulfated oxysterol has been shown to be a potent regulator of lipid metabolism in human hepatocytes (Ren S et al., Biochem Biophys Res Comm 2007, 360, 802) and in macrophages (Ma Y et al., Am J Physiol Endocrinol Metab 2008, 295, 1369).
Many other sterol mono-, di-, and trisulfates have been described in invertebrates from warm waters.
Annasterol is a sterol monosulfate which was isolated from Poecillastra laminaris, a sponge living in Philippines seawater. It has potent antibacterial properties against Bacillus vulgaris (De Riccardis F et al., Tetrahedron Lett, 1992, 33, 1097).
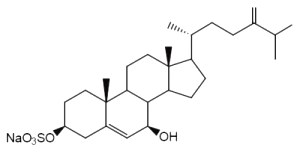
Annasterol
Weinbersterols are sterol disulfates isolated from Petrosia weinbergi, a sponge living in Bahamas waters. One of these forms is shown below. They all have antiviral properties against the virus of cat leukemia and against VIH (Sun H H et al., Tetrahedron, 1991, 47, 1185).
.
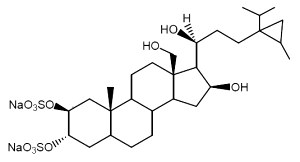
Weinbersterol A
About a dozen of sterol trisulfates have been described. They are all found in sponges and have the sulfate groups in the same position : carbon 2, 3 and 6. Some tested molecules have shown interesting antibacterial and antiviral properties (Mc Kee TC et al., J. Med. Chem., 1994, 37, 793). Several of these sulfated sterols may be found in the D’Auria’s paper (D’Auria MV et al., Chem Rev 1993, 93, 1839).
One of these compounds, isolated from the sponge Pseudoaxinissa digitata (Demospongia, order Axinellida) and showing anti-HIV activity is shown below.
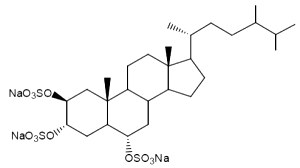
Lembehsterols have similar structures, they were isolated from the marine sponge Petrosia strongylata. These sterols showed inhibitory activity against thymidine phosphorylase, which is an enzyme related to angiogenesis in solid tumors (Aoki S et al., Chem Pharm Bull 2002, 50, 827).
These molecules are currently used as models for chemists who are trying to increase their antiviral potency in modifying molecule structures.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire