
HALOGENATED FATTY ACIDS
This page is intended as a comprehensive and rapid survey of fatty acids possessing carbonâhalogen covalent bonds, which have been described in living organisms, some of them being formed during chemical reactions in various industrial situations. Halogenated fatty acids which are one of the most interesting groups among the naturally occurring halogen compounds are not well known but several reviews on these compounds may be found. Halogenated fatty acids are found in different groups of organisms from microorganisms to the highest plants and animals. As halogen, they contain one or several atoms of fluor, chlorine, or bromine.
The principal fluorinated component (3% of the seed oil) was detected in a west African shrub (Dichapetalum toxicarium, Chailletaceae) and identified as a w-fluorooleic acid (18:1n-9) (Peters RA et al. Biochem Pharmacol 1959, 2, 25). D. toxicarium has also the ability to accumulate fluoroacetate in the leaves.
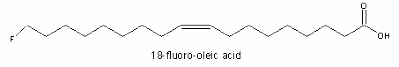
The toxicity observed in sheep eating the D. toxicarium seeds was explained by the metabolism of the fluoro acids which produces the very toxic fluoro-acetic acid.
Later, other fluorinated compounds were identified : w-fluorocapric (C10:0), w-fluoromyristic (C14:0), and w-fluoropalmitic acids (Peters RA et al., Nature 1960, 187, 573). A reexamination of the seed oil after saponification and using mass spectrometry established in addition the presence of w-fluoro derivatives of palmitoleic (C16:1), stearic (C18:0), linoleic (C18:2), and eicosenoic (C20:1) acids (Hamilton JTG et al., Chem Phys Lipids 2000, 105, 93). Details on these fluorinated fatty acids may be found in several reviews (Harper DB et al., Nat Prod Rep 1994, 11, 123; Dembitsky VM et al., Prog Lipid Res 2002, 41, 315).
An usual w-fluoro-9,10-epoxy C18 fatty acid was identified together with other w-fluoro acids of 16:1, 18:0, 18:1, 18:2, 20:0 and 20:1 in the seed oil of Dichapetalum toxicarium (Dichapetalaceae)(Hamilton JTG et al., Phytochemistry 1997, 44, 1129).
Various halogenated fatty acids were chemically synthesized (review by Dembitsky VM et al., 2002).
A series of fluorine-containing unsaturated fatty acids, which are potential fungicides, have been prepared (Michel D et al., Synthesis 1996, 1007).
These compounds have been found to be the major constituents among organohalogens in fish, molluscs, invertebrates and seaweeds.
The presence of six isomeric acids, 9-Cl-9-, 10-Cl-9-, 9-Cl10-, 10-Cl-9, 11-Cl-12-, and 12-Cl-11-hydroxystearic acids were reported for the first time in jellyfish (Auritia aurita) (White RH et al., Biochemistry 1977, 16, 4944).
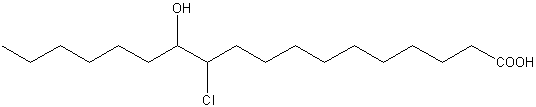
11-Cl,12-OH-stearic acid from Auritia aurita
All compounds, as the previous one, with adjacent chloro- and hydroxy- goups are named fatty acid chlorohydrins.
Later, several dichloro-tridecanoic, -hexadecanoic, and -octadecanoic acids were isolated from fish, mussel, lobster, or jellyfish.
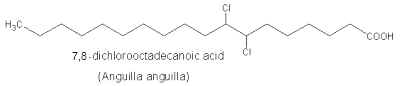
The occurrence of a tetrachloro-fatty acid was reported in fish lipids.
The formation of fatty acid chlorohydrins by the addition of HOCl to unsaturated fatty acids is well known (Winterbourn CC et al., Arch Biochim Biophys 1992, 296, 547). The reaction of HOCl in vitro with a variety of fatty acids and phospholipids (mainly plasmalogens) has been well studied (Wang WY et al., Anal Biochem 2013, 443, 148). Oleic acid yields approximately equal amounts of the 2 positional isomers of the 9,10-monochlorohydrin, while linoleic acid can form both monochlorohydrins and at higher HOCl/lipid ratios, bis-chlorohydrins (4 isomers are possible). With polyunsaturated fatty acyl chains, such as arachidonic, eicosapentaenoic and docosahexaenoic acids, increasingly complex mixtures of chlorohydrins can be formed. In phospholipids, such as glycerophosphocholines, multi-chlorinated products are formed, and increasing modification results in a greater tendency to generate lysophospholipids by hydrolytic cleavage of the modified fatty acyl chain (Arnhold J et al., Biochim Biophys Acta 2002, 1572, 91). These reactions suggest that the formation of lysophospholipids from unsaturated phospholipids by hypochlorous acid can be relevant in vivo under acute inflammatory conditions.
Chlorinated fatty acids are also formed during the production of bleached paper for oxidation of lignins using elemental chlorine. As chlorinated oleic and linoleic acids are found in the wood, several dichloro- and tetrachloro-acids are found in bleached pulp mill effluents. Similar compounds may be formed also during bleaching of wheat flour and during the disinfection process of drinking water.
A review of these fatty acids and their derivatives was made by Dembitsky VM (Prog Lipid Res 2002, 41, 315).
The first investigation of bromine in fatty acids was reported in the 1970s in lipid extracts from marine animals (Lunge G, JAOCS 1972, 49, 44).
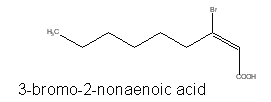
Several brominated fatty acids, such as 3-bromo-2-nonaenoic acid, have been isolated from red marine algae (McConnell O et al., Phytochemistry 1980, 19, 233).
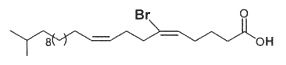
Brominated fatty acids are rare in higher plants. Two brominated stearic acids, such as 9,10-dibromo-octadecanoic and 9,10,12,13-tetrabromo-octadecanoic acids, were isolated from the seed oil of the Asian plant Eremostachys molucelloides (Dembitsky VM, Prog Lipid Res 2002, 41, 315).
Lichens are unique symbiotic organisms that produce different brominated acetylenic acids such as 18-bromo-octadeca-5,17-diene-15-ynoic acid (Rezenka T et al., Phytochemistry 1999, 50, 97). Several other bromoallenic fatty acids have been found in lichen species. A short review on unusual brominated fatty acids may be found by Dembitsky VM (Inform 2003, 14, 30).
A bromoallenic acid has been characterized in lipids from lichens and may be used as chemotaxonomic purposes (Rezanka T. et al., Phytochemistry 2001, 56, 869). This triene compound is hydroxylated in 15 and brominated in 18.
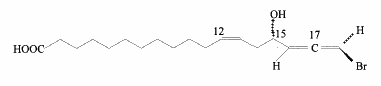
Similar molecules were described in sponges living in different locations and were named "demospongic acids". They have been shown to have good antimicrobial activity (Hirsh S et al., Tetrahedron 1987, 14, 3257).
A series of brominated polyunsaturated long-chain fatty acids (C20-C28) has been isolated from different species of marine invertebrates (anemone, sponge) (Review in Dembitsky VM, Prog Lipid Res 2002, 41, 315).
These unusual fatty acids were found in tropical marine sponges (Amphimedon terpenensis) and their structure elucidated by efficient techniques (Garson MJ et al., Lipids 1993, 28, 1011). The three fatty acids studied have a common scheme with the following structure:
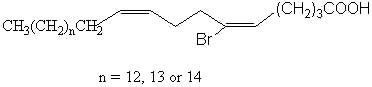
Fractionation of lipid extracts of a marine sponge Homaxinella sp led to the isolation of a new brominated fatty acid which was found in the free form or acylating a glycerol molecule. Physical studies revealed it has the structure of a branched-chain demospongic acid. The compound showed only moderate cytotoxicity (Mansoor TA et al., Lipids 2005, 40, 981).
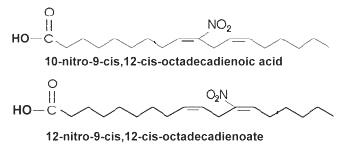
Nitration of unsaturated fatty acids by nitric oxide-derived species (peroxinitrite, nitrous acid …) has been first demonstrated for linoleic acid (O’Donnell VB et al., Chem Res Toxicol 1999, 12, 83). It has been shown later that two positional isomers of nitrolinoleic acid (10- and 12-nitro-9,12-octadecadienoic acid) are present in plasma lipoproteins and red cell membranes (about 500 nM, partly esterified to phospholipids or neutral lipids) (Baker PRS et al., PNAS 2004, 101, 11577).
Mass spectrometric analysis of human plasma and urine revealed abundant nitrated derivatives of all principal unsaturated fatty acids. Nitrated palmitoleic, oleic, linoleic, linolenic, arachidonic, eicosapentaenoic and docosahexaenoic acids were detected in concert with their nitrohydroxy derivatives (Baker PR et al., J Biol Chem 2005, 280, 42464).The nitration of linoleic acid is likely produced in vivo by the initiation of its auto-oxidation by nitric oxide (.NO2) by means of hydrogen abstraction from bis-allylic carbon followed by a reaction with .NO2. An acid-catalyzed nitration is also possible during precise physiological or pathological situations or in vitro in the presence of HNO2– radical. mechanisms. Moreover, nitrohydroxyarachidonate has been shown to exhibit vaso-relaxatory effects in vitro, an observation consistent with its production (Balazy M et al., J Pharm Exp Ther 2001, 299, 1).
It was previously demonstrated that these nitrated fatty acids may serve as cell signaling molecules transducing the vascular actions of .NO (Lim DG et al., PNAS 2002, 99, 15941). Further experiments proved that nitroalkene derivatives of linoleic acid are pluripotent signaling mediators that act directly via receptors and also by transducting the signaling actions of .NO (Schopfer FJ et al., J Biol Chem 2005, 280, 19289). Furthermore, nitrolinoleic acid was shown to be a potent endogenous ligand for peroxisome proliferator-activated receptor gamma (PPARg) (Schopfer FJ et al., PNAS 2005, 102, 2340). Several experimental results provide mechanisms to explain nitric oxide production from nitrated lipids, mechanisms which support the role of lipid sources of nitric oxide in endothelium-independent vasorelaxation (Lima ES et al., Free Rad Biol Med 2005, 39, 532). Nitro-oleic acid was shown to lower blood pressure in a hypertension model in wild-type mice, but was ineffective in mutant mice (Charles RL et al., PNAS 2014, May 19). That protection from hypertension afforded by the oleic acid-rich Mediterranean diet is likely mediated by a nitro-fatty acid-dependent inhibition of soluble epoxide hydrolase, thus preventing the hydrolysis of epoxyeicosatrienoic acid which induces vasodilation.
Several observations have indicated that nitrolinoleic and nitrooleic acids belong to a class of endogenous anti-inflammatory mediators (Cui T et al., J Biol Chem 2006, 281, 35686). They inhibit cytokine and inducible nitric oxide synthase expression in lipopolysaccharide (LPS)- and interferon-g-stimulated monocytes. A nitro-fatty acid (nitro-oleic acid), naturally produced during myocardial ischaemia, was shown to have anti-inflammatory properties and could have therapeutic actions against myocardial ischaemia/reperfusion injury (Rudolph V et al., Cardiovasc Res 2009, cvp275).
Reviews on the mechanisms of formation, chemical characterization, and biological properties of nitrated fatty acids may be consulted with interest (Trostchansky A et al., Free Rad Biol Med 2008, 44, 1887; Freeman BA et al., J Biol Chem 2008, 283, 15515; Rubbo H et al., Biochim Biophys Acta 2008, 1780, 1318). The biochemical interactions between nitric oxide and lipid oxidation pathways have reviewed (O’Donnell VB et al., Circ Res 2001, 88, 12).
In plants, a transcriptomic analysis by RNA-seq technology established in Arabidopsis a clear signaling role for nitrolinolenic acid, demonstrating that it was involved in plant defense response against different abiotic-stress conditions, mainly by inducing heat shock proteins and supporting a conserved mechanism of action in both animal and plant defense processes. A review of these topics may be consulted (Mata-Pérez C et al., Plant Physiol 2016, 170, 686).
ARSENIC CONTAINING FATTY ACIDS
Arsenic containing fatty acids were reported for the first time in cod-liver oil (in Rumpler A., Angew. Chem. Int. Ed 2008, 47, 2665). These compounds may be considered as true arsenolipids in contrast with commonly called "arsenolipids" which are only lipid-soluble arsenicals. Total arsenic analyses on fish oils from various origins gave concentrations from 4.3 to 10.5 mg As per kg (Schmeisser E et al., The Analyst 2005, 130, 948). Six arsenic-containing fatty acids have been identified (Rumpler A et al., Angew Chem Int Ed 2008, 47, 2665). Among them, a homologous series of arsenic-containing saturated fatty acids of the type (CH3)2As(O)-(CH2)nCOOH (n = 12, 14, 16, and 18) with a dimethylarsinoyl group was determined. Two further unsaturated compounds are likely analogous to oleic acid (18:1 n-9) and 7,10,13,16,19-docosapentaenoic acid (22:5 n-3) commonly found in cod-liver oil. Two of these compounds are shown bellow.
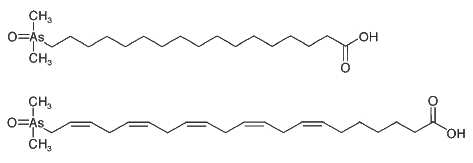
The toxicological relevance of these arsenic-containing fatty acids in cod-liver oil remains to be evaluated.
A review of occurrence and chemistry of arsenolipids in marine oils and fats have been reviewed (Sele V et al., Food Chem 2012, 133, 618).
Very recently, the synthesis of four arsenic-containing fatty acids was reported. In that work, bis(dimethylarsenic) oxide was reacted with brominated compounds (Taleshi MS et al., Organometallics 2014,33,1397). The synthesis of two new arsenic-containing fatty acids and their identification infish by liquid chromatography coupled simultaneously to mass spectrometry has been reported (Arroyo-Abad U et al., Eur J Lipid Sci Technol 2016, 118, 445).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire