
OLIGOGLYCOSYLCERAMIDES
Glycosphingolipids containing more than one sugar moiety belongs to the oligoglycosylceramide group. These highly glycosylated compounds are found in animals and plants.
Digalactosyl ceramides are found in brain. They are also present in the kidney of patients with Fabry’s disease (Sweeley CC et al., J Biol Chem 1963, PC 3148). They are also present in sea snails and insects (Hori T et al., Prog Lipid Res 1993, 32, 25).
The lactosyl ceramide (lactose = glucose + galactose) was isolated in spleen (Klenk E et al., Z Physiol Chem 1942, 273, 253) and erythrocytes (Klenk E et al., Z Physiol Chem 1953, 295, 164). Up to four glycosyl groups are found in some tissue cerebrosides (kidney, lung, blood) where they have immunochemical properties. Lactosyl ceramide was shown to be specifically coupled with the Src family kinase Lyn in plasma membrane microdomains of human neutrophils (Sonnino S et al., Glycoconj J 2009, 26, 615).
The triglycosyl ceramide was described in erythrocytes, spleen and liver and was shown to be also accumulated in Fabry’s disease. According to the old nomenclature, triglycosyl ceramides are known as globotriaosyl ceramide. They are also described in molluscs and in insects (Hori T et al., Prog Lipid Res 1993, 32, 25). In human, Fabry disease, an X-linked disorder of glycosphingolipid catabolim, results in an accumulation of a globotriaosylceramide, Gal-Gal-Glc-Cer or Gb3, throughout various cells, organs and tissues. The detection of that glycolipid in urine may help in the diagnosis of the disease (Rozenfeld PA et al., Clin Chim Acta 2009, 403, 194). It has been demonstrated that the expression of Pk blood group antigen, which is a globotriaosylceramide, strongly influences the susceptibility to HIV-1 infection. That antigen which is a new endogenous cell-surface factor, may provide protection against HIV-1 infection. (Lund N et al., Blood 2009, 113, 4980). Experiments have demonstrated the utility of using a completely synthetic, water soluble globotriaosylceramide analogue, for possible future use as a novel therapeutic approach for the systemic treatment of HIV/AIDS (Harrison AL et al., Glycoconj J 2010, 27, 515).
Accumulation of ceramide di- and trihexoside in the Fabry’s disease in man leads to kidney failure.
The tetraglycosyl ceramide is found specifically in human erythrocytes and described as a N-acetylgalactosaminyl-galactosyl-galactosyl-glucosyl ceramide (Klenk E et al., Z Phys Chem 1951, 288, 220), the compound was given the name globoside (Yamakawa T et al., J Biochem 1952, 39, 393) which still is commonly used.
An unusual tetraglycosyl ceramide was described in a Gram-negative bacteria, Sphingomonas paucimobilis (Kawahara K et al., FEBS Lett 1991, 292, 107). In contrast with all other Gram-negative bacteria which carry lipopolysaccharide in their outer membrane, this species has only a complex glycosphingolipid. This lipid is based on a ceramide linked to a glycosyl portion consisting of a mannose-galactose-glucosamine-glucuronic acid tetrasaccharide. The ceramide part is formed of hydroxymyristic acid (2 hydroxy-14:0) amide-linked to a dihydrosphingosine residue (which may have a cyclopropyl ring).
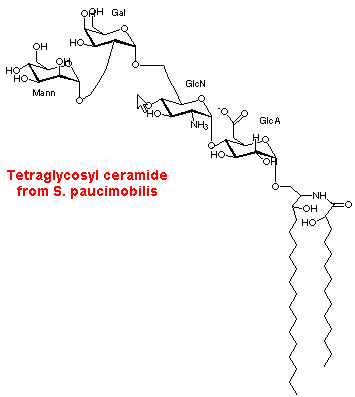
In plants, these glycolipids are found located in the lamellar membranes of chloroplasts but without recognized function.
A great variety of glycosylceramides with up to 9 sugar moieties have been described in insects (Hori T et al., Prog Lipid Res 1993, 32, 25).
The presence of a fucolipid carrying Lewis system antibodies was reported in human tumors (Hakomori EL et al., Biochim Biophys Acta 1970, 202, 225). The complete structure of a similar compound extracted from human intestine was described as a pentaglycosyl ceramide containing fucose linked to N-acetylglucosamine (Smith EL et al., J Biol Chem 1975, 250, 6059). The ceramide was shown to contain phytosphingosine as the major long chain base liked to a hydroxy fatty acid (C16 to C25 carbon atoms).
On the basis of chemical analysis and immunological activity, the following structure was proposed :
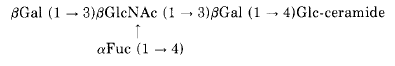
Several blood group fucolipids and their isomers were described in human and canine intestine (McKibbin JM et al., J Biol Chem 1982, 257, 755). Some of them contain two fucose groups in the molecular structure.
It has been shown that neutral fucosylated glycolipids of the ganglio series, containing polyunsaturated fatty acids, are required for male fertility in mice (Sandhoff R et al., J Biol Chem 2005, 280, 27310). One of these glycolipids has the following structure :
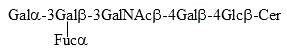
These glycolipids contain polyunsaturated very long chain fatty acids (C26-C32 and 4 to 6 double bonds. Later, it was shown that these fucolipids are relevant for proper completion of meiosis (Rabionet M et al., J Biol Chem 2008, 283, 13357).
Several tetra- and penta-hexosyl ceramides containing fucose were described in adult cestodes and their plerocercoid larvae. Compound A (see the figure below) was described in plerocercoids of the parasite Spirometra erinacei (Kawakami Y et al., J Biochem 1993, 114, 677). The same structure was also found in Diphyllobothrium hottai adult worms whereas the structure B was found in plerocercoids of that species (Iriko H et al., Eur J Biochem 2002, 269, 3549). The ceramide contained sphinganine or 4-hydroxysphinganine and non-hydroxy fatty acids with carbon atoms ranging from 16 to 28.
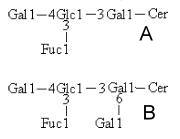
The sequence Gal beta-4 Glc beta-3 Gal was commonly found in glycosphingolipids from cestodes and was named spirometo series, and the name spirometosides was proposed for glycosphingolipids having this carbohydrate sequence (Kawakami Y et al., Eur J Biochem 1996, 239, 905). That structure may have taxonomic significance, being characteristic of pseudophyllidean tapeworms. It was also shown that spirometosides may be involved in host-parasite interaction (Iriko H et al., Eur J Biochem 2002, 269, 3549).
Several penta- and hexa-hexosyl ceramides containing fucose were described in molluscs (Hori T et al., Prog Lipid Res 1993, 32, 25).
An extensive review of relationships between structure and function of glycosphingolipids may be consulted with interest (Hakomori S, Biochim Biophys Acta 2008, 1780, 325).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire