
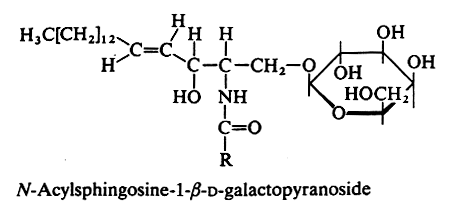
MONOGLYCOSYLCERAMIDES
An amide bound between a fatty acid and the amine group of sphingosine or other related amino alcohols gives rise to a ceramide. Attachment of one sugar group by O-ester linkage to the primary alcohol of the ceramide yields a ceramide hexoside.
In animals the galactocerebroside (most commonly known as cerebroside, or GalCer) is the glycosphingolipid the most frequently found (in brain tissue). The fatty acid (with 20 to 24 carbon atoms) is normal or 2-hydroxylated (mainly in kidney and brain), the long-chain base being sphingosine or dihydrosphingosine.
An acetylated derivative of GalCer (3-O-acetyl-sphingosine GalCer) has been characterized in rat brain myelin (Dasgupta S et al. J Lipid Res 2002, 43, 751). That is, an acetyl group is linked at the C3-OH of the sphingosine base.
Cerebrosides were the first glycosphingolipids discovered in brain tissue and named by L.W. Thudichum in 1874. He demonstrated that they are composed of a fatty acid, a long-chain base and a sugar. The hexose component was identified as galactose by Thierfelder (Z Physiol Chem 1890, 14, 209).
The production of corresponding a-anomers in mammals have not been described so far, but it has been proposed that at very low levels a-GalCer may be present and necessary for invariant natural killer T cell homeostasis (Kain L et al., Immunity 2014, 41, 543). Similar compounds with a a-galactosyl linkage have been described in the marine sponge Agelas mauritianus (Natori T et al., Tetrahedron Lett 1993, 34, 5591). These glycolipids, named agelasphins, are formed by a ceramide containing a C24 hydroxylated fatty acid and phytosphingosine-like base with 16 to 19 carbon atoms. All these glycolipids showed antitumor activity. KRN7000 is a synthetic analog of an agelasphin. It is a specific ligand for human and mouse natural killer T cells (NKT cells) and remains the best studied ligand of the lipid-binding MHC class I-like protein CD1d. It protects against LPS-induced shock and displays potent antitumor activity in various in vivo models. The potential of a-GalCer stimulated iNKT cells in tumor treatment is actively investigated.
Cerebrosides are concentrated in nervous tissues where they increase from about 0.02% of the dry weight in human fetal brain to adult levels of 2% in gray matter and 12% in white matter.
Cerebrosides were also shown to be present in larval and adult forms of the tapeworm, Spirometra erinacei (Kawakami Y et al., Lipids 1995, 30, 333). The hexose consisted primarily of galactose, the sphingoid base was sphinganine (d18:0) or phytosphingosine (t18:0) and the fatty acid ranged from 16 to 30 carbon atoms, hydoxy stearic acid being also found.
A glucosylceramide (glucocerebroside) was first isolated in 1940 from the spleen of a man with Gaucher’s disease (Halliday N et al., J Biol Chem 1940, 132, 171). Later, it was isolated in several extra-neural organs. In erythrocytes glucose is detected. It was also shown to be present consistently in multiple-drug resistant cell lines where it may hold significance for the early identification of drug-resistant tumors (Lucci A et al., Anticancer Res 1998, 18, 475).
A phylogenetic dichotomy of nerve glycosphingolipid has been established in studying nervous tissues from a wide variety of animals (Okamura N et al., Proc Natl Acad Sci 1985, 82, 6779). It appears that nerves of protostome animals contain only glucocerebrosides while galactocerebrosides are mainly present in deuterostome animals. This correlation suggests that an evolutionary trend which corresponds with the genesis of highly structured myelin around axons in deuterostomes.
Cerebroside (monohexoside) storage in excessive amounts in the brain leads to Gaucher’s disease characterized by spleen and liver enlargement and mental retardation.
Since 1988, it has been realized that cerebrosides self-aggregate in cellular membranes to form a separate phase that is less fluid than the bulk phospholipids based on diacylglycerol. Sphingolipid-based microdomains or rafts were originally proposed to sort membrane proteins along the cellular pathways of membrane transport (Simons K et al., Biochemistry 1988, 27, 6197). Presently, most excitement focuses on their organizing functions in signal transduction (Brown DA et al., J Biol Chem 2000, 275, 17221). The importance of rafts and translocation of sphingolipids have been reviewed (Van Meer G et al., J Biol Chem 2002, 277, 25855).
Glycosphingolipids like galactosylceramides are used as cellular binding sites for a wide variety of pathogens, including viruses, bacteria, fungi and parasites (see web site).
Original glucocerebrosides (renierosides) have been studied in a marine sponge, Reniera sp. These forms were shown to have amide-linked long-chain hydroxylated fatty acid moieties (C25, C26 or C28) and a sphingoid base with three double bonds (Mansoor T et al., J Nat Prod 2007, 70, 1481).
In plants, the existence of glycosphingolipids was documented only in 1954 (Carter HE et al., J Biol Chem 1954, 206, 613). In plants, glucose is found instead of galactose, the fatty acid is most frequently hydroxylated (2-hydroxy with 16 or 18 carbon atoms) and the long-chain base is phytosphingosine or dehydrophytosphingosine. Monoglucosecerebrosides containing 4,8-sphingadienine have been described in lipid extracts from soybean and nuts of almond (Prunus amygdalus)(Shibuya H et al., Chem Pharm Bull 1990, 38, 2933; Sang S et al., J Agric Food Chem 2002, 50, 4709). These compounds have been reported to exhibit significant biological activities, such as anti-ulcerogenic, ionophoretic and anti-hepatotoxic activities. A glucosphingolipid based on dehydrophytosphingosine and hydroxylated fatty acid of various length has been described in an Euphorbiaceae (Euphorbia sororia) (Zhang WK et al., Chem Phys Lipids 2007, 148, 77). This compound has neuritogenic activity as other parent compounds isolated from mushroom (Qi JH et al., Tetrahedron 2001, 56, 5835).
A monoglucosecerebroside (pinelloside) with strong antimicrobial properties (against Gram-positive and -negative bacteria and against fungi) was described in the tuber of Pinella ternata (Araceae), one component of decoctions used in traditional Chinese medicine (Chen JH et al., Phytochemistry 2003, 64, 903). Its structure was shown to include a glucose moiety and the unusual 4,11-sphingadienine linked to a 2-hydroxy-palmitic acid. Fungal glucosylceramides can be considered non only as a structural component of cell membranes but participate also in recognition by the immune system, regulation of virulence and in cellular signaling (Nimrichter L et al., Lipid Insights 2008, 2, 61).
A glycosylcerebroside with a tri-hydroxylated C18 sphingosine analogue linked to a C17 hydroxylated fatty acid has been described as a potential biomarker for viral infection of planktonic coccolithophore populations (Vardi A et al., Science 2009, 326, 861). It was shown that it was able to induce biochemical hallmarks of programmed cell death in uninfected host, Emiliana huxleyi.
Several glucocerebrosides have been isolated from the aerial parts of Orostachys japonicus (Crassulaceae) (Zhang H et al., Food Chem 2012, 131, 1097). The most active molecule in inhibiting fatty acid synthase has the fatty acid and sphingosine moieties which were determined as 2-hydroxyeicosa-6,9-dienoic acid and 2-amino-1,3,4-trihydroxytetracosa-6,10-diene, respectively. Its cytotoxic activity may provide a scientific basis for the folk remedy using the plant to treat cancer in China and Japon.
Several glucosphingolipids have been described in wheat bran, some of them having interesting cytotoxic effects against colon cancer cells (Zhu Y et al., J Agric Food Chem 2013, 61, 866). These sphingolipids may be implicated in colon cancer prevention as a component of this food frequently considered as bioactive.
It appears progressively that plant sphingolipids are composed of structurally diverse molecules that are important as membrane components and bioactive molecules. An appreciation of the relationship between structural diversity and functional significance of plant sphingolipids is emerging through characterization of Arabidopsis mutants coupled with a lipidomics approach (Markham JE et al., Curr Opin Plant Biol 2013, 16, 350).
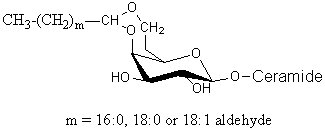
Glycosphingolipids having a long-chain cyclic acetal have been isolated from human brain and named plasmalocerebroside (Levery SB et al., Biochemistry 1992, 31, 5335). They were found as spots having much higher mobility than unmodified cerebrosides on thin-layer chromatography. These components were found to be fatty aldehyde conjugates of cerebroside, characterized by the formation of either 3,4 or 4,6 cyclic acetal linkages to the b-galactopyranosyl residue. This acid-labile structure is analogous to plasmalopsychosine. The yield of this compound amounted to 0.3 mg per Kg (wet weight) of brain tissue (4000 times lower than the concentration of galactocerebroside). Only the galactocerebroside 4,6-O-cyclic fatty acetal is shown below.
There are several acidic glycosphingolipids in the outer bacterial membrane of the Sphingomonadaceae which are recognized by T cell antigen receptors on the mouse natural killer T cells, and thus an innate-type immune response towards glycosphingolipid-containing bacteria (Kinjo Y et al., Chem Biol 2008, 15, 65). One of the most abundant of these acidic glycosylceramides is the a-galacturonosyl ceramide (Wu D et al., PNAS 2006, 103, 3972). This glycolipid is a weaker NKT cell agonist than a-galactosylceramide.
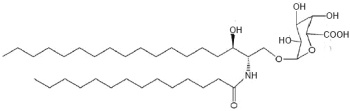
a-Galacturonosyl ceramide
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire