
DITERPENES
They have 20 carbon atoms and are derived from geranylgeraniol pyrophosphate.
They are of fungal or plant origin and are found in resins, gummy exudates, and in the resinous high-boiling fractions remaining after distillation of essential oils. The rosin remaining after distilling pine turpentine, for instance, is rich in diterpenoids. In ancient times, conifer exudates were used for caulking boats and waterproofing ropes. Resin secretion is also recognized to be part of the resistance mechanism conifers employ against bark beetles and their associated pathogenic fungi. Diterpenoid groups that are physiologically active include: vitamin A activity (retinol), phytohormones that regulate plant growth and germination, e.g. gibberellin, fungal hormones that stimulate the switch from asexual to sexual reproduction, e.g. trisporic acid; disease resistance agents (phytoalexins), e.g. casbene and podocarpic acid, the anticancer drug, taxol, from the bark of the yew tree, the cancer promoter, phorbol, and natural cannabinoids.
The diterpenes have exceptionally open chain, as found in geranylgeraniol or phytol which forms a part of chlorophyll and the side chain of vitamin E and K, and crocetin which is a diacid diterpenoid and the lipid part of the crocins, glycosylated derivatives present in saffron.
Examples of diterpene substances are given below :


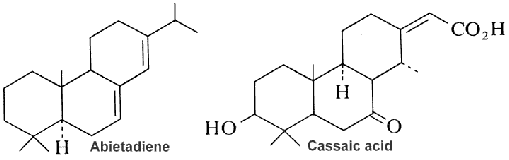



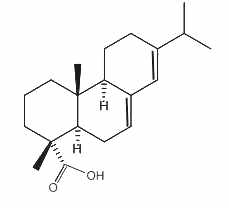
Abietic acid
Cembrene (the simplest form of the cembranoid family) is a type of macrocyclic diterpene consisting of
four isoprene units bonded âhead to tailâ. It was firstly found in the oleoresin secreted from the trunk of a pine tree.
All cembranoids feature a 14-carbon macrocyclic skeleton. Cembranoids are primarily distributed in plants of the Nicotiana and Pinus genera, as well as in some marine organisms (soft coral). About 90 naturally occurring cembranoids have been isolated from tobacco. The chemical structures, biosynthesis, and bioactivities of tobacco cembranoids have been reviewed (Yan N. et al. Indust Crops Prod 2016, 83, 66).
Abietic acid is an irritant compound present in pine wood and resin. It is the most abundant compound present in rosin, the solid fraction of the oleoresin of coniferous trees. It is mainly used to make lacquers and varnishes and metal resinates. These resinates are produced in reacting abietic acid (or a similar compound) with a metal salt (gold, indium, nickel, palladium, platinum, silver …) and used in a wide variety of applications where high purity metals in organic solution form is needed (gravure printing inks, vitrifiable colors, antifouling agent, dryers for paints and varnishes …).
Tanshinones are abietane diterpenes, isolated mainly from Salvia miltiorrhiza (Lamiaceae), a plant largely used in traditional Chinese medicine for the treatment of cardiovascular and inflammatory diseases. Among them, tanshinone I is an apoptose inductor and displays several anticancerous biological properties (Y. Dong et al., Nat Prod Rep 2011, 28, 529). Several analogues have been synthesized for clinical trials.

Tanshinone I
Steviol is the aglycone of stevia‘s sweet glycosides, one of them being formed by replacing one hydrogen atom (bottom) with glucose via an ester link, and another hydrogen atom (top) with a disaccharide (glucose and rhamnose). The steviol glycosides are responsible for the sweet taste of the leaves of the stevia plant (Stevia rebaudiana, Asteraceae). These compounds are 40 to 300 times sweeter than sucrose. They are developed to be used in sweet drinks.
Steviol
Retene is present in tars obtained by distillation of resinous wood, it is an important pollutant eliminated by the paper factories. This diterpene is present in geological sediment where it is formed by diagenesis from abietic acid, several intermediates having been recognized (Wakeham SG et al., Geochem Cosmochim Acta 1978, 42, 289). Thus, with cadalene (sesquiterpene), retene, a diterpenoid dehydrogenation product, is used in paleobotanic to estimate the importance of ancient pine forests.
Retene
Gibberellins are a family of compounds, over 130 members exist whose structures and occurrence can be found on the web. The most important in plants is gibberellin A1 which is responsible for stem elongation. The most widely available compound is gibberellic acid (one double bond in the right cycle). Among the physiological properties, gibberellins are involved in stem growth, seed germination and fruit setting and growth.
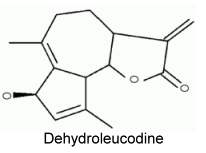
Dehydroleucodine was isolated from Artemisia douglasiana, a popular medicine in Argentina and was shown to have several physiological and therapeutic properties : anti-proliferative activity in G2 phase (Lopez ME et al., Protoplasma 2002, 219, 82), cytoprotective agent for gastric ulcers and a general antioxidant.
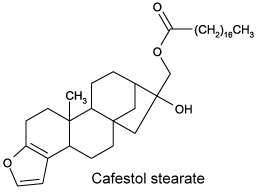
Cafestol and kahweol are present in high concentrations (up to 18% diterpene esters) in the oil derived from coffee beans. The only difference between cafestol and kahweol is an extra double bond present in the second cycle of kahweol. These diterpenes are esterified with one fatty acid (C14 to C24), palmitic and linoleic acids being the major esterified fatty acids (Folstar P et al., Lebensm Wiss Technol 1975, 8, 286). Terpenes were also shown to induce an elevated plasma cholesterol content in human (Boekschoten MV et al Nutr J 2003, 2, 8).
Phorbol is a diterpene isolated in 1934 from croton oil (seeds of Croton tiglium). Various fatty acid esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C as they mimic diacylglycerols. The most common phorbol ester is phorbol-12-myristate-13-acetate (PMA), which is used as a research tool in models of carcinogenesis.

Phorbol
R : myristic acid (C14:0), Ac : acetate group
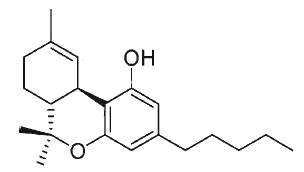
Cannabinoids are a group of diterpenes present in Cannabis (Cannabis sativa L). All these substances are structurally related to tetrahydrocannabinol (THC) and are able to bind to specific cannabinoid receptors.
Tetrahydrocannabinol, also known as Δ9-THC or Δ9-tetrahydrocannabinol, is the main psychoactive substance found in the plant. It was isolated by Mechoulam R et al. in 1964 (review in Mechoulam Ret al., Chem Phys Lipids 2000, 108, 1).
Δ9-Tetrahydrocannabinol (THC)
Some diterpènes (Kanakugiol, Kingidiol) present in various plants have been shown to disrupt the receptor complex of insect juvenile hormone (Lee SH et al., PNAS 2015, 112, 1733). These results indicate that newly discovered compounds could be used as the starting material for development of novel insecticides.
Andrographolide is a diterpene of the labdane family isolated from the stem and leaves of Andrographis paniculata (Acanthaceae, native to India and Sri Lanka). It has been studied for its effects on cell signaling, immunomodulation, and stroke. A study has shown that A. paniculata plant extract has anti-diabetic properties, in accordance with its local use for that disease. Andrographolide has also the property to help recover the cognitive decline in a rodent model of in a natural model of Alzheimerâs disease (Rivera DS et al., Neurobiol Aging 2016, 46, 204). These important results suggest that Andrographolide could be used in human as a potential therapy for Alzheimerâs disease.

Andrographolide
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire