
SESTERTERPENES
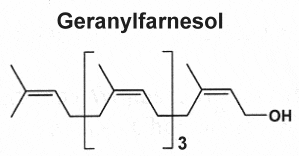
They are derived from geranylfarnesol pyrophosphate and have 25 carbon atoms (the sester- prefix means half to three, i.e. two and a half.). These relatively rare lipids were first isolated from insect protective waxes and from fungal sources. Now, they are known to be widespread, they have been isolated from terrestrial fungi, lichens, higher plants, insects, and various marine organisms, especially sponges.
They exhibit diverse biological properties such as anti-inflammatory, cytotoxic, anticancer, antimicrobial, and anti-biofilm activities. The structure of the most recent sesterterpenoids and their activities have been reviewed (Wang L et al., Nat prod Rep 2013, 30, 455).
Three examples of sesterterpenes are shown below.
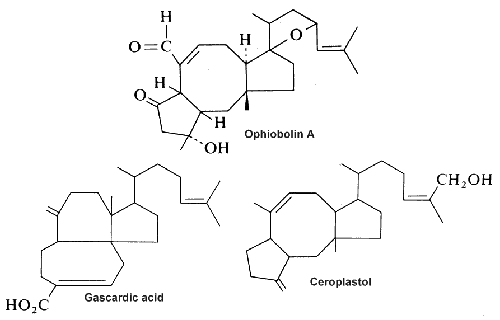
Ophiobolin A, a fungal metabolite and a phytotoxin stimulates the net leakage of electrolytes and glucose from maize seedling roots and was found to be a potent inhibitor of calmodulin-activated cyclic nucleotide phosphodiesterase (Leung PC et al., J Biol Chem 1984, 259, 2742-7).
Gascardic acid is the first sesterterpenes that was isolated in 1965 from the secretion of an insect, Gascardia madagascariensis.
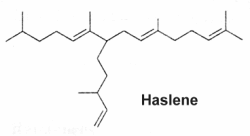
Variously unsaturated and branched sesterterpenes, known as Haslenes, were found in species of diatomaceous algae (Volkman JK at el., Org Geochem 1994, 21, 407). They have 25 carbons and 3, 4 and 5 double bonds. They are widely distributed and abundant in marine sediments. Several haslenes were found to be produced by a pennate diatom Haslea ostrearia according to the culture temperature and were shown to have cytostatic properties (Rowland SJ et al., Phytochemistry 2001, 56, 597). One of them, with only one double bond and known as IP25, is shown below.
C25 monounsaturated isoprenoid hydrocarbon (IP25)
This compound has been detected in sea ice in Canadian Arctic and was shown to be a specific biomarker for studying the Quaternary Arctic ice history (Belt ST et al., Org geochem 2007, 38, 16). The monitoring of this lipid appears valuable for reconstruct paleo-sea ice distributions and accurate calibration of climate prediction models (Stein R et al., Org Geochem 2013, 55, 98). The occurrence and variable abundance of several di- and tri-unsaturated C25 haslenes as biomarkers in Antarctic marine sediments have been proposed as a possible proxy measure of paleo sea-ice extent in the Southern Ocean (Smik L et al., Org Geochem 2016, 95, 71).
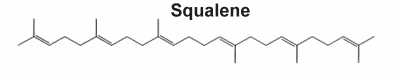
They form a large group of natural products which includes steroids and consequently sterols, they derived from C30 precursors. Nearly 200 different triterpene skeletons are known from natural sources and represent structurally cyclization products of Squalene which is the immediate biological precursor of all triterpenoids. In 1934, Robinson proposed a direct cyclization of squalene to the steroid molecule. In 1936, Nobel laureate researcher Paul Karrer described the biochemical structure of squalene for the first time. It has major specificities, which are related to anticancer properties, the maintenance of the oxidation/antioxidation balance, and its antiaging capabilities (review in Ronco AL et al., Funct Foods Health Dis 2013, 3, 462). Shark liver oil is considered the richest source of squalene, accounting for at least 40% of its weight. It is also widely distributed in nature, in lesser proportions in amaranth oil (6-9%), in wheat germ oil, and in olive oil (usually from 0.4% up to 1% in extra virgin olive oil). It has been shown that the human body synthesizes an average amount of 1.5 g/day (Liu GC et al., J Lipid Res 1976, 17:38).
Biochemistry, molecular biology, and applications of squalene (Spanova M et al., Eur J Lipid Sci Technol 2011, 113, 1299), and the origin of their skeletal diversity (Xu RX et al., Phytochemistry 2004, 65, 261) have been reviewed.
Squalene epoxide (2,3-oxidosqualene) is produced by the enzyme squalene epoxidase which use NADPH and oxygen to oxidize squalene. This metabolic step is the first in sterol biosynthesis leading to the formation of lanosterol or cycloartenol.
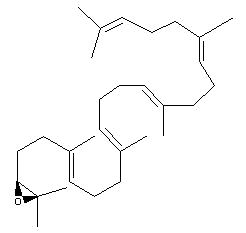
Squalene epoxide
Squalane is a completely saturated derivative of squalene. Present in sebum, it is largely used as a component in many cosmetic products. It is obtained by hydrogenation of squalene extracted from olive oil.
The large group of steroids, including sterols, are present in very small amounts in bacteria but at larger amounts in plants and animals while the hopanoids are very abundant in prokaryotes where they replace cholesterol.
Triterpenoids are widely distributed in edible and medicinal plants and are an integral part of the human diet. They are being evaluated for use in new functional foods, drugs, cosmetics and healthcare products. Screening plant material has identified fruit peel and especially fruit cuticular waxes as promising and highly available sources (Szakiel A et al., Phytochem Rev 2012, 11, 263). Amyrin isomers are the predominant compounds in tomato fruit wax, with quantities that vary considerably in different cultivars and during the subsequent stages of fruit development. A methyl ether derivative of an amyrin isomer, miliacin, has been proposed as a sedimentary molecular tracer of the history of Panicum (broomcorn millet) cultivation (Bossard N et al., Org Geochem 2013, 63, 48).
Among the large number of triterpenoid structures, some of them are shown below.
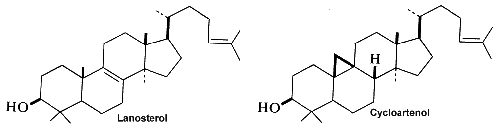
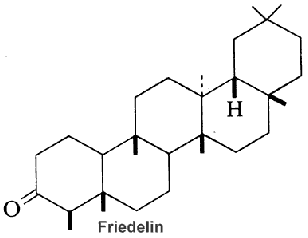
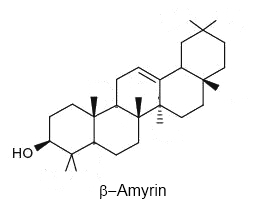
Serratane-type compounds constitute an important group of pentacyclic triterpenes with an unusual seven carbons ring. They are synthesized through the cyclisation of bis-epoxy-squalene and not from epoxy-squalene, the precursor of more common pentacyclic triterpenes (Xu R et al., Phytochemistry 2004, 65, 261).
More than 100 different serratenoids (serratenes and derivatives have been reported, most having been identified in club mosses, conifers, and in few angiosperms (Primulaceae and Leguminosae).
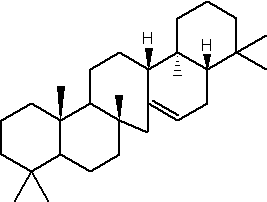
Serratene
As opposed to other serratenoids, methoxy-serratenes appear constrained to conifers, and more specifically to Pinaceae (mainly Pinus, Picea) (Le Milbeau C et al., Org Geochem 2013, 55, 45). In addition to diterpenoids, they potentially constitute a new powerful tool for palaeoenvironmental reconstruction.
Steroids are modified triterpenes which derived also from squalene by cyclization, unsaturation and substitution. The nucleus of all steroids is the tetracyclic C17 hydrocarbon 1,2-cyclopentanoperhydrophenanthrene (gonane or sterane) substituted by methyl groups at C10 and C13, as well as an alkyl side-chain at C17. Steroids may possess a nucleus derived from the former one by one or more C-C bond scissions or ring expansions or contractions.
Gonane and three examples of basic unsubstituted steroids are shown below.
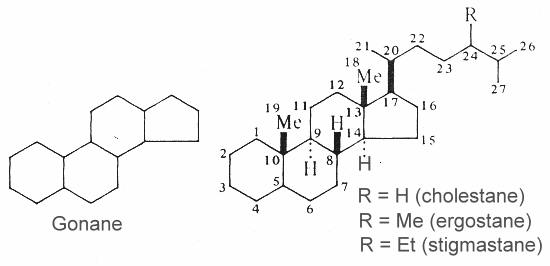
Unsaturated steroids with most of the skeleton of cholestane containing a 3b-hydroxyl group and an aliphatic side chain of 8 or more carbon atoms attached to position 17 form the group of sterols.
The hopanoids are pentacyclic triterpenoids based on the hopane skeleton (with a five-membered ring E) are widely distributed in prokaryotes but were not detected in Archaea (Rohmer M et al., J Gen Microbiol 1984, 130, 1137). They are often considered bacterial sterol analogs and were first isolated from resins of tropical trees of the genus Hopea by the Ourisson’s team. In most cases, the hopanoid content of the cell is comparable with the cholesterol content of eukaryotic cells. They are considered as membrane rigidifiers. Furthermore, they are the precursors of several derived compounds (homohopanoids) in sediments and oils (Ourisson G et al., Pure Appl Chem 1979, 51, 709) and thus could be considered as the most abundant natural products on earth (Ourisson G et al., Accounts Chem Res 1992, 25, 398). Although oxygen is not required for hopanoid biosynthesis, the vast majority of known hopanoid producers are aerobic or micro-aerophilic bacteria (Rohmer M et al., J Gen Microbiol 1984, 130, 1137). Thus, hopanoids are found predominantly in aerobic methanotrophs, heterotrophs and cyanobacteria, but have also been found in some anaerobic bacteria (Sinninghe Damsté JS et al., Org Chem 2004, 35, 561).
The simplest C30 hopanoid is diploptene.
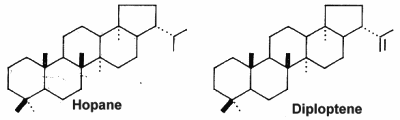
Cyanobacteria are presently the only known bacteria to synthesize abundant 2-methylhopanoids with an extended polyhydroxylated side chain. The most abundant hopanoids are C35 bacteriohopanepolyols in which the side-chain of the parent structure contains a variable number of vicinal hydroxyl groups
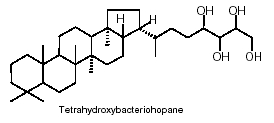
The hopanoids are widely distributed in bacteria and blue-green algae where they are important cell membrane constituents. It is often said that hopanoids are the "most abundant natural products on Earth".
As hopanoids are very stable and are not easily degraded, they are receiving an intense attention as biological markers with applications for geochemical studies of petroleum source rocks and oils. These biomarkers are mainly derived from bacterial bacteriohopanepolyols (biohopanoids). The 2-methylhopane derivatives are abundant in organic-rich sediments as old as 2,500Myr. These lipids may help constrain the age of the oldest cyanobacteria and the advent of oxygenic photosynthesis. They could also be used to quantify the ecological importance of cyanobacteria through geological time (Summons RE et al., Nature 1999, 400, 554). More recent investigations have shown that the production of substantial quantities of polyhydroxylated 2-methylhopanoids may occur in phototrophs other than cyanobacteria and that their biosynthesis does not require molecular oxygen (Rashby SE et al., PNAS 2007, 104, 15099). Hopanoids, and especially their C-2 methylated derivatives, have been found in high abundance in membranes of several plant symbionts (Bradyrhizobium, Frankia). In these bacteria, they contribute to growth and protection against stresses (Kulkarni G et al., mBio 2015, 6(5):e01251-15). In some symbiotic bacteria, an unusual lipopolysaccharide bearing a hopanoid covalently linked to lipid A has been described (Silipo A et al., Nat Commun 2014, 5:5106). a brief review of the hopanoid biosynthesis may be found in the Sohlenkamp paper (Sohlenkamp C et al., FEMS microbiol Rev 2016, 40, 133).
Aminohopanoids not very different from the previous one have been identified in several methylotrophic bacteria (Neunlist S et al., Biochem J 1985, 231, 635). One of the most abundant is a aminohopane pentol, mixed with other parent compounds (triol and tetrol). This C30 aminopentol is a possible precursor of the widespread C29 hopanoid chemical fossils.
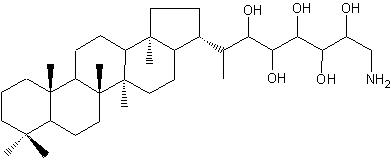
Aminobacteriohopane
Different bacterial groups possess recognizable biohopanoid distributions, giving hopanoids marker potential for specific bacterial populations and environmental conditions. More than 150 individual hopane derivatives have been isolated from various types of sedimentary organic matter.
Other pentacyclic triterpenoids based on the lupane skeleton include a very large number of naturally occurring members with different functional groups which are found in vegetables and fruit. Among them, lupeol is one of the most ubiquitous compounds.
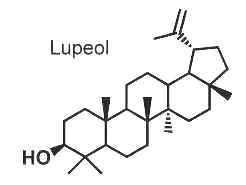
Lupeol was shown to have various pharmacological properties, inducing anti-inflammatory and anti-arthritic responses and inhibition of tumor growth (Saleem M et al., Cancer Res 2005, 65, 11203) in animal cells.
Long-chain fatty acid esters (C20, C22 and C26) of lupeol extracted from an African plant, Holarrhena floribunda (Apocynaceae), were shown to have strong antimalarial activity, especially against drug-resistant strains of Plasmodium falciparum (Fotie J et al., J Nat Prod 2006, 69, 62).
Fatty acid esters (palmitic or stearic acid) of lupeol have been isolated from green propolis produced by honeybees from vegetative apices of the Asteraceae Baccharis dracunculifolia from Brazil (Furukawa S et al., Chem Pharm Bull 2002, 50, 439). These compounds were named procrim a and b.
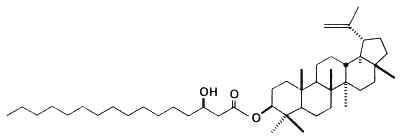
Palmitic acid ester of lupeol (Procrim a)
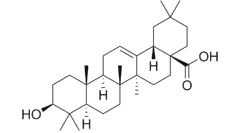
Ursolic acid is present in numerous plants and is the best known triterpene from the ursane group. It amounts at more than 1 g per 100 g dry weight in Thymus, Rosmarinus, Salvia, Lavandula, and Eucalyptus. It is found at high concentration in coffee seeds and in apple fruit (mainly in apple peels). It is used in cosmetics as an anti-inflammatory, antibacterial and antifungal drug. Ursolic acid is also able to inhibit some form of cancer, particularly multiple myeloma (Pathak AK et al., Mol Cancer Res 2007, 5, 943).
Ursolic acid
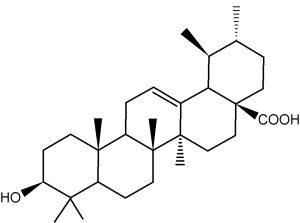
A compound very similar to ursolic acid, oleanolic acid, is widely distributed in food and medicinal plants.
It is hepatoprotective and exhibits antitumor and antiviral properties. It is the main constituent of grape berry cuticular waxes (Szakiel A et al., Phytochem Rev 2012, 11, 263). Its presence could be at the origin of the numerous health benefits, including protection against heart disease, ascribed to the moderate consumption of wine. Oleanolic acid and another parent compounds, maslinic acid, are concentrated in olive cuticular waxes, representing 31â44 % and 55â68 % of total wax extract, respectively. These compounds could be related to the numerous health-promoting properties, such as anticancer, antihyperglycemic and antiparasitic activities, reported for olive consumption (Szakiel A et al., Phytochem Rev 2012, 11, 263).
Oleanolic acid
A parent compound of oleanolic acid (oleanonic acid), with a ketone group instead of the hydroxyl group, is present in Pistachia resin and has been used to establish that this substance was used in the preparation of pharaonic meat mummies (Clark KA et al., PNAS 2013, 110, 20392).
As ursolic acid but in contrast with the hopane series, which possesses a five-membered ring E, the gammacerane skeleton is characterized by a six-membered ring (see below).
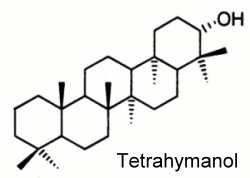
Tetrahymanol (gammaceran-21a-ol), a typical representative of the series, was first isolated from the ciliate protozoan Tetrahymena pyriformis (Mallory FB et al., J Am Chem Soc 1963, 85, 1362). Later, it was detected in a number of other eukaryotes, e.g. in ferns, fungi and some other ciliates. Its occurrence was long thought to be restricted to eukaryotes but its presence in sediments pointed out a much more widespread distribution in living organisms. The finding of tetrahymanol in the purple nonsulfur bacterium Rhodopseudomonas palustris opened new insights into the biochemistry of these molecules in bacteria (Kleemann G et al., J Gen Microbiol 1990, 136, 2551). Tetrahymanol and novel methylated homologues were discovered in nitrogen-fixing bacterium Bradyrhizobium japonicum (Bravo JM et al., Eur J Biochem 2001, 268, 1323).
Gammacerane structures were shown to be reliable geochemical indicators for water column stratification in marine or in lacustrine deposits (Sinninghe Damste JS et al., Geochim Cosmochim Acta 1995, 59, 1895).
Celastrol is a hopanoid triterpene extracted from a plant used in the Chinese medicine, Tripterygium wilfordii. That plant is useful to treat inflammatory and autoimmune diseases. New research have demonstrated that it is a potent proteasome inhibitor and can help in the treatment of prostate cancer (Yang H et al., Cancer Res 2006, 66, 4758). Several biochemical properties bring about several investigations in the field of cancer treatment or prevention. Its antioxidant and anti-inflammatory properties has been the basis of research on diseases induced by monocytes and macrophages activation, including neurodegenerative process (Allison AC et al., Prog Neuropsychopharmacol Biol Psychiatry 2001, 25, 1341).
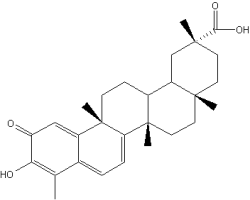
Celastrol
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire