
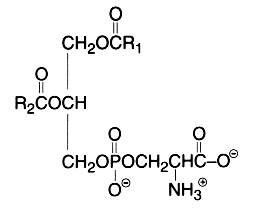
The main component of this group is a diacyl form: 1,2-diacyl-sn-glycero-3-phospho-L-serine or phosphatidylserine. It is the only amino acid-containing glycerophospholipid in animal cells.
Folch isolated a preparation of phosphatidylserine about 92% pure from brain "cephalin" by means of solvent fractionation and determined its exact composition (J Biol Chem 1948, 174, 439). This phospholipid occurs quite widely in nature but usually in concentrations less than 10% of the cell phospholipid pool. In brain tissue, myelin (white matter) has the highest amounts. Phosphatidylserine has generally highly unsaturated acyl chains.
From the formula it appears clearly that phosphatidylserine possesses three ionizable groups: a diester phosphoric acid, an amino group and a carboxyl function. Thus at pH 7, the phosphate and the carboxyl functions are in anionic form and the amino group is positively charged. When isolated, this lipid contains 1 mol of a cation (K or Na) which can be removed by washing the solvent with 0.05 N HCl.
Phosphatidylserine was not shown to be involved in cell signaling through the formation of metabolites (as phosphatidylcholine or phosphatidylinositol) but is a key component in the activation of various kinases (kinase C, Raf-1 kinase) as well as the blood coagulation process. Thus, externalization of phosphatidylserine from the inner to the outer membrane leaflet of platelets allows coagulation factors to bind and form enzyme/cofactor complexes and aids complement binding (Heemskerk JW et al., Thromb Haemost 2002, 88, 186). Loss of this ability to externalize phosphatidylserine (and phosphatidylethanolamine) is characteristic of the rare bleeding disorder Scott syndrome (Zwaal RF et al., Cell Mol Life Sci 2005, 62, 971).
For this last effect, it has been shown that both the PS head-group per se and unsaturation of the 1,2 fatty acids are important (Smirnov MD et al., Biochemistry 1999, 38, 3591). Later, two molecular species (18:0/20:4 and 18:0/18:1) have been shown to be the molecular determinants that regulate coagulation (Clark SR et al., PNAS 2013, 110, 5875).
However, lyso derivatives of phosphatidylserine were detected after corneal injury and may be involved in maintaining the integrity of the normal cornea in promoting cellular regeneration (Liliom K et al., Am J Physiol 1998, 274, C1065).
Phospatidylserine with ether linkage (1-0-alkyl glycerophosphoserines) were identified in the human lens (Deeley JM et al., Anal Chem 2009, 81, 1920). This is the first report of these phospholipids in human tissue.
Serine plasmalogens have been described in marine bivalves (Kraffe E et al., Lipids 2004, 39, 59). They were found to be enriched with non-methylene-interrupted fatty acids (mainly 7,17-22:2).
Phosphatidylserine is located entirely on the inner layer of the plasma membrane. This normal distribution is altered during platelet activation and cellular apoptosis. Thus, it has been shown that phosphatidylserine is exposed on the surface of apoptotic cells (Fadok VA et al., J Immunol 1992, 148, 2207) and on the nuclei expelled from erythroid precursor cells (Yoshida H et al., Nature 2005, 437, 754). This signal works as an "eat me" signal for phagocytes. Further investigations have shown that a transmembrane protein (Tim4) is the phosphatidylserine receptor for the engulfment of apoptotic cells (Miyanishi M et al., Nature 2007, 450, 435).
A growing body of evidence indicates the involvement in apoptotic cells of oxidized phosphatidylserine as pattern recognition ligands for scavenger CD-36 receptors on macrophages (Greenberg ME et al., JEM 2006, 203, 2613).
The regulation of a pro-apoptotic factor, caspase-3, by phosphatidylserine rich in DHA was demonstrated in a model of neuronal cells ( Akbar M et al., Proc Natl Acad Sci 2005, 102, 10858). This mechanism may contribute to neurological deficits associated with n-3 fatty acid deficiency and support protective effects of DHA in pathological models such as brain ischemia or Alzheimer’s disease.
Phosphatidylserine was shown to modulate the activity of several key enzymes involved in cellular signaling. The detection of the movement of phosphatidylserine across membranes may be visualized using a complex of annexin V fused with a green fluorescence protein (Calderon F et al., J Neurochem 2008, 104, 1271).
The formation and the multiple functions of phosphatidylserine in mammalian cells have been reviewed (Vance JE et al., Biochim Biophys Acta 2013, 1831, 543).
A N-acylphosphatidylserine has been described in lipid extracts of mouse brain (Guan Z et al., Biochemistry 2007, 46, 14500). The complexity of this phospholipid is further increased by the presence of diverse amide-linked N-acyl chains, which include saturated, monounsaturated, and polyunsaturated species. N-Acylphosphatidylserine was also detected in the lipids of pig brain, mouse macrophage tumor cells, and yeast. It may be a biosynthetic precursor of N-acylserine which may have mediator functions.
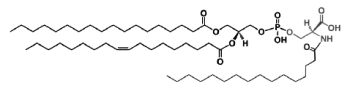
N-Acylphosphatidylserine
A graphical chart of the metabolism of phosphatidylserine may be found on the BioCarta web site.
A derivative of phosphatidylserine, a phosphatidylserylglutamate, was isolated from Escherichia coli (Garrett TA et al., J Lipid Res 2009, 50, 1589). This minor lipid component has a polar head group formed by a serylglutamate residue. Several species with various fatty acids were discovered, the main one being R1
= 16:0 and R2 = 18:1n9.
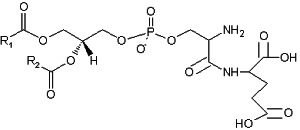
Phosphatidylserylglutamate
Lysophosphatidylserine has long been known as a signaling lipid in mast cell biology, markedly enhancing histamine release (Smith GA et al., FEBS Lett 1979, 105, 58) and eicosanoid production. More recently, there has been a resurgence of interest in lysophosphatidylserine as new roles in the promotion of phagocytosis of apoptotic cells have been studied. An important review of the actually known roles of lysophosphatidylserine may be consulted (Frasch SC et al., Prog Lipid Res 2012, 51, 199).
The phosphoglyceride phosphatidyl-O-N-(2-hydroxyethyl) glycine has been isolated from the brown algae Fucus serratus (Eichenberger W et al., J Plant Physiol 1995, 146, 398).
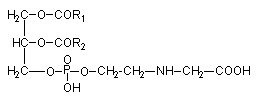
It was shown that in that Phaeophyceae the fatty acid composition (R1 and R2) was about 80% arachidonic acid (20:4n-6) and 10% 20:5n-3. A large survey proved that this phospholipid was present in 30 different species representing the 16 orders of brown algae and in amounts of 8-25 mol% of total phospholipids. Furthermore, this lipid is very likely a specific constituent of Phaeophyceae, since it has not been observed in green algae or vascular plants so far.
This simplest polyol-phospholipid was first isolated in 1958 (Benson et al., Biochim Biophys Acta 1958, 27, 189) who described its structure. It can be defined as 1,2-diacyl-sn-glycero-3-phospho-1′-sn-glycerol.
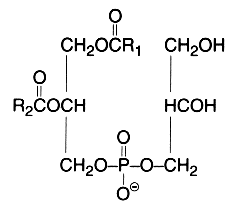
This lipid occurs widely but very low amounts are found in animal tissues (mainly in mitochondria), in plants it forms 20 to 30 % of total phospholipids (mainly in the chloroplast). In bacteria, trace amounts up to 70% of the total lipids are found. A dialkyl analogue forms a small proportion (about 5%) of the phospholipids from halophilic bacteria but a great proportion of the archaeal virus membranes (Vitale R et al., Biochim Biophys Acta 2013, 1831, 872). Its acidic properties require the use of acid mixtures for the extraction to prevent the tight association of the lipid with cations in the cell. Phosphatidylglycerol is the second most abundant phospholipid in lung surfactant since it is present at 715% of the total phospholipid. Its precise biological role in lung is unknown but it may play a role in alveolar stability and recent studies suggest that it regulates the innate immune response (Numata M et al., Proc Natl Acad Sci USA 2010, 107, 320).
An acylated form (the fatty acid being linked to the second glycerol) has been described, acyl phosphatidylglycerol, which is found in bacteria (Corynebacterium amycolatum) as a minor component (Yagüe G et al., FEMS Microbiol. Lett. 1997, 151, 125). The acyl group on the glycerol was only octadecenoyl acid. Acyl phosphatidylglycerol can be considered as a useful chemical marker for the identification of C. amycolatum in addition to the absence of mycolic acids. That phospholipid was also described in lipid extracts from a vegetal (oat, Avena sativa) where a high content of N-acylphosphatidylethanolamine was also observed (Holmback J et al., Lipids 2001, 36, 153).
A phosphorylated form (at the 3-position of the second glycerol), known as phosphatidyl glycerophosphate is found in halophilic bacteria (and in archaeal viruses) but is also an intermediate in the biosynthesis of diphosphatidylglycerol.
The existence of lysyl-phosphatidylglycerol was discovered in 1965 (Gale EF et al., Biochem J 1965, 94, 390)) and its metabolism described in 1971 in Staphylococcus aureus (Short SA et al., J Bacteriol 1971, 108, 219). Later, this compound was described in polar lipids of group B Streptococci (Fischer W, Biochim Biophys Acta 1977, 487, 89), in Caulobacter crescentus (Jones DE et al., Can J biochem 1979, 57, 424), in Bacillus subtilis (Deutsch RM et al., J Biol Chem. 1980, 255, 1521), and was shown to be a major component in Staphylococcus aureus and S. intermedius (Nahaie MR et al., J Gen Microbiol 1984 130, 2427). It was also detected in Vagococcus fluvialis (Fisher W et al., J Bacteriol 1998, 180, 2950), in several species of Listeria (Fisher W et al., Int J Syst Bacteriol 1999, 49, 653) and in Clostridium novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193). Thus, lysyl-phosphatidylglycerol is now a well-known membrane lipid in several gram-positive bacteria but is almost unheard of in gram-negative bacteria. It has been suggested that this phospholipid derivative may selectively protect bacteria against antimicrobial polypeptides (Ganz T, J Exp Med 2001, 193, F31).
An alanyl derivative of phosphatidylglycerol has been first discovered in Clostridium welchii (Macfarlane MG, Nature 1962, 196, 136), in C. novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193) and in many gram-positive bacteria (OLeary WM et al., In "Microbial Lipids". C. Ratledge et al., ed. Vol. 1. Acad Press, New York, NY. pp.117201). Presumably, isomers carry the aminoacyl residue at different positions (O-1 or O-2) of the glycerol moiety.
Diphosphatidylglycerol
(Cardiolipin)
Also referred historically to cardiolipin, this curious lipid is found almost exclusively in mitochondria and in bacteria. It can account for as much as 20% of mitochondrial lipids. Cardiolopin was first discovered in beef heart tissue (Pangborn MC, J Biol Chem 1942, 143, 247) but, later, it was recognized to be not specific to the heart.
This phospholipid was discovered in 1906 by Wasserman through its antigenic properties in attempting to isolate the substance that confers on alcoholic extracts of beef heart the property of reacting with sera from cases of syphilis (Wassermann test) (Wassermann A et al., Dtsch Med Wochenschr 1906, 32, 745). A popular serological test, similar to the Wassermann reaction for detection of syphilis, used a mixture of cardiolipin, phosphatidylcholine and cholesterol (for details on antiphosphospholipid antibodies see the review of McIntyre JA et al. (Prog Lipid Res 2003, 42, 176 ). Since 1911, it is known that the antigenic activity is associated with the acetone-insoluble portion of the heart extract. Mrs Mary C. Pangborn was the first to "isolate and purify a serologically active phospholipid from beef heart". She proposed to designate this compound "cardiolipin" (heart lipid). In her first report (Pangborn M., Proc Soc Exp Biol Med 1941, 48, 4 84) she claimed that :
| "A new phospholipid from beef heart has been isolated and purified. On hydrolysis it yields fatty acids and a phosphorylated polysaccharide. The name cardiolipin is suggested for this substance, which is essential for the reactivity of beef heart antigens in the serologic test for syphilis" |
Later, she improved and simplified the preparation methodology (Pangborn MC, J Biol Chem 1944, 153, 343). She reported that the previous observation of a carbohydrate component in the molecule was an error "apparently due to persistent traces of carbohydrate impurities". At that time, the purification was entirely based on solvent properties (methanol, ethanol, acetone, ether, benzene, ethyl acetate, chloroform, water) and barium salt insolubility, but no information on the composition of this lipid was given.
Evidence that cardiolipin is composed of three glycerol, two phosphoric acid and four fatty acid residues was brought in 1958 by MacFarlane MG et al. (Biochem J 1958, 70, 409, Nature 1958, 182, 946). One year later, she proposed the exact position of the fatty acids (R in the figure below) in the cardiolipin molecule (Nature 1959, 180, 1808). This discovery was possible thanks to the report of a diacylglycerol liberation from a phospholipid fraction in hot acetic acid (Coulon-Morelec MJ et al., C R Acad Sci, Paris 1958, 246, 1936). The mechanism of this reaction was then studied and the liberation of the diacylglycerol was shown to be linked to the presence of a free hydroxyl adjacent to the phosphoric acid, condition present in cardiolipin and phosphatidylinositol (Coulon-Morelec MJ et al., Bull Soc Chim Biol 1960, 42, 867).
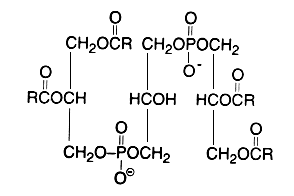
This phospholipid can be defined as 1,3-bis(sn-3-phosphatidyl)-sn-glycerol. Curiously, mammalian cardiolipin contains up to 90 mol% of one fatty acid, linoleic acid (Hoch FL, Biochim Biophys Acta 1992, 1113, 71).
The most abundant cardiolipin species from various organisms and tissues (human heart, human lymphoblasts, rat liver, Drosophila, sea urchin sperm, yeast, vegetal cells) contained only one or two types of fatty acids, which generated a high degree of structural uniformity (Schlame M et al., Chem Phys Lipids 2005, 138, 38). In contrast, it was demonstrated the presence of changes in the cardiolipin molecular species profile in the mammalian brain during the perinatal period. These changes are correlated to the massive alterations in neuronal remodeling and apoptosis that occur after birth (Cheng H et al., Biochemistry 2008, 47, 5869). Over 100 molecular species have been identified. The embryonic cardiolipin profile was notable for the presence of abundant amounts of relatively short fatty chains. After birth, a more complex range of molecular species, rich in arachidonic and docosahexaenoic acids, was observed. Similarly, cardiolipin compositional abnormalities involving abundance of immature molecular species (shorter chain saturated or monounsaturated fatty acids) have been observed in brain tumor mitochondria, results which support the Warburg theory of cancer (Kiebish MA et al., J Lipid Res 2008, 49, 2545).
In some marine bivalves (Pecten maximus, Crassostrea gigas and Mytilus edulis), cardiolipin molecules are found predominently with four docosahexaenoyl chains (22:6 n-3) and are presumed to reflect a specific adaptation to environmental conditions (Kraffe E et al. Lipids 2002, 37, 507). Cardiolipin from a Manila clam, Ruditapes philippinarum, was shown to contain EPA and DHA in approximately equal proportions and contributing together up to 73% of the total fatty acids of that phospholipid (Kraffe E et al., Lipids 2005, 40, 619). A survey of the cardiolipin fatty acid composition among thirty-five species of marine mollusks revealed a control and a conservation of that composition in species of the same phylogenetic group (Kraffe E et al., Lipids 2008, 43, 961).
Although some microorganisms lack cardiolipin, it comprises 2-25% of lipid phosphorus in the majority of bacteria. Several species contain up to 38% (Bacillus subtilis), 59% (Streptococcus) and even 79% (Staphylococcus aureus). A diphytanylglycerol ether analogue of cardiolipin has been isolated from the purple membranes of Halobacterium salinarum (Corcelli A et al., Biochemistry 2000, 39, 3318). An important fraction of cardiolipin plasmalogen (with one or two alk-1′-enyl chains) has been reported in lipid extracts of Clostridium novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193).
A lipidomic approach of two haloalkaliphilic archaeal microorganisms of the genus Natronococcus has yielded detailed information on novel cardiolipins based on 2-O-sesterpanyl-3-O-phytanyl- sn-glycerol (C25, C20) diether lipid cores (Angelini R et al., Biochim Biophys Acta 2012, 1818, 1365). The presence of two C25 and two C20 lipid chains (Zip structure) in this phospholipid of haloalkaliphilic archaea could make more stable the membrane, conferring the ability to better withstand high pH gradients in the presence of very high salt concentrations (De Rosa M. et al., J Gen Microbiol 1982, 128, 343).
This phospholipid has been extensively studied since it was found to be associated to cytochrome oxidase in the electron transport system located in the mitochondrial cristae membranes, in chloroplast thylakoid membranes, and in bacterial membranes (review in Robinson NC, J Bioenerg Biomembr 1993, 25, 153). Cardiolipin is emerging as an important factor in the regulation of mitochondrial bioenergetics in that it interacts with several vital inner membrane proteins, including anion carriers and respiratory chain complexes. Although its exact function remains to be defined, it seems to be a potential factor in several pathologies, such as thyroid disease (Paradies GFM et al., Arch Biochem Biophys 1993, 307, 91), oxidative stress (Iwase HT et al., Biochem Biophys Res Comm 1996, 222, 83), and aging (Paradies GFM et al., FEBS Lett 1997, 406, 136). It has been demonstrated that mitochondrial dysfunction associated with cardiac ischemia-reperfusion is associated with cardiolipin oxidation. Melatonin was able to protect against such dysfunction and oxidative alteration of cardiolipin (Petrosillo G et al., FASEB J 2006, 20, 269). Accumulating evidence suggests that this unique lipid also has active roles in several of the mitochondria-dependant steps of apoptosis (cytochrome c dissociation from the mitochondrial inner membrane, permeabilization of the mitochondrial outer membrane) (Gonzalvez F et al., Apoptosis 2007, 12, 877). The complex role of cardiolipin in human health and disease has been reviewed (Claypool SM et al., Tr Biochem Sci 2012, 37, 32). A review of the relationship between cardiolipin and lipid intakes and pathophysiology in human may be consulted (Feillet-Coudray C et al., Cah Nutr Diet 2015, 50, 331).
The fluorescent dye 10-N-nonyl acridine orange is extensively used for location and quantitative assays of cardiolipin in living cells on the assumption of its high specificity for cardiolipin (Garcia Fernandez MI et al., Anal Biochem 2004, 328, 174; Kaewsuya P et al., Anal Bioanal Chem 2007, 387, 2775).
It was shown that a lyso precursor of cardiolipin (monolysocardiolipin) could be used as a specific marker for the Barth Syndrome (an X-linked recessive disorder) when measured in patient fibroblasts (van Werkhoven MA et al., J Lipid Res 2006, 47, 2346). The analysis of monolysocardiolipin by HPLC-MS was shown to be a powerful tool to diagnose patients with clinical signs of Barth syndrome among those who suffer from cardiomyopathy with unknown etiology (Houtkooper RH et al., Anal Biochem 2009, 387, 230). The differential diagnosis of these patients includes any male child who has neutropenia, dilated cardiomyopathy with endocardial fibro-elastosis, and abnormal mitochondria in the heart. These patients may also exhibit idiopathic cardiomyopathy with growth retardation as well as 3-methylglutaconic aciduria.
This increase in monolysocardiolipin content is accompanied by a decrease in tetra-linoleyl species of cardiolipin (review in: Hauff KD et al., Prog Lipid Res 2006, 45, 91). These defects could be the result of mutations in the gene TAZ which control the production of proteins known as tafazzins.
A large review on the biosynthesis and functional role of cardiolipin was proposed by Schlame M et al. (Prog Lipid Res 2000, 39, 257). Its role in mitochondrial metabolism has been reviewed in 2008 (Houtkooper RH et al., Cell Mol Life Sci 2008, 65, 2493; Klingenberg M, Biochim Biophys Acta 2009, 1788, 2048). Its organization, its role in ATP synthesis and in Barth’s syndrome has been released (Haines TH, Biochim Biophys Acta 2009, 1788, 1997).
As phosphatidylglycerol, it must be extracted from tissue homogenates using acidic solvents and some precautions are needed to prevent its loss in aqueous solutions.
Cardiolipin has been identified in quite all mammalian tissues (not found in erythrocytes, skin…) where it constitutes 2-10% of total phospholipids. It occurs also in invertebrates, plants, algae, yeast and bacteria. The presence of cardiolipin in prokaryotes was used to support the hypothesis of the bacterial origin of mitochondria in eukaryotes.
Alanylcardiolipin was isolated and characterized from a streptococci, Vagococcus fluviatilis (Fisher W et al., J Bacteriol 1998, 180, 2093). This phospholipid was shown to contribute up to 38% of the lipid phosphorus. Similarly, a lysyl derivative of cardiolipin has been described first in several species of Listeria, a gram-positive bacteria (Peter-Katalinic J et al., J Lipid Res 1998, 39, 2286) where it could be valuable as chemotaxonomic marker. In that phospholipid, the hydroxyl group of the middle glycerol moiety is esterified with a lysyl residue.
A glucosylated cardiolipin was described in several strains of Streptococcus (Fisher W, Biochim Biophys Acta 1977, 487, 74).
This curious phospholipid known also as bis(monoacylglycerol)phosphate has a stereochemical configuration, 3-acyl-sn-glycero-1-phosphoryl-1′-sn-[3′-acylglycerol], different from that of all the other known mammalian phospholipids, that have the sn-glycero-3-phosphoryl configuration (Brotherus J et al., Chem Phys Lipids 1974, 13, 178).
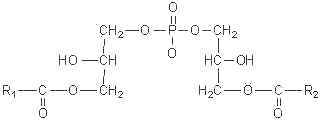
This phospholipid was first identified in the lung of mammals (Body DR et al., Chem Phys Lipids 1967, 1, 254) and was later shown to be enriched in lysosomes of rat liver (Wherrett JR et al., J Biol Chem 1972, 247, 4114). Its accumulation was demonstrated in the lysosomal compartment, especially during some lysosomal storage disorders (Niemann-Pick diseases, neuronal ceroid lipofuscinoses) and drug-induced phospholipidosis. Its involvement in both dynamics and lipid/protein sorting functions of late endosomes has started to be documented, especially in the control of cellular cholesterol distribution (Hullin-Matsuda F et al., Prost Leukotr Essent Fatty Acids 2009, 81, 313). Investigations in animals with gangliosidoses have shown that the content of bis(monoacylglycerol)phosphate in brain was significantly greater in humans and in animals with either GM1 or GM2 ganglioside storage diseases, than in brains of normal subjects (Akgoc Z et al., J Lipid Res 2015, 56, 1006). C22:6, C18:0, and C18:1 were the predominant fatty acids in gangliosidosis brains.
In addition, R1 or R2 are frequently arachidonic acid (alveolar macrophages) or docosahexaenoic acid in some other cells (Holbrook PG et al., Biochim Biophys Acta 1992, 1125, 330), oleic acid being the other acyl group.
More recently, it was shown that lysobisphosphatidic acid is a major phospholipid, with phosphatidylcholine, in the late endosomes (Kobayashi T et al., J Biol Chem 2002, 277, 32157). It was also found that lysobisphosphatidic acid exhibited unique pH-dependent fusogenic properties, thus, this lipid is an ideal candidate to regulate the dynamic properties of these cellular membranes.
It was reported that lysobisphosphatidic acid containing two 22:6n-3 may be a marker of drug-induced phospholipidosis (Baronas ET et al., Toxicol Appl Pharmacol 2007, 218, 72). Initially, its level was shown to account for 21% of the increase in phospholipids in a rat model of phospholipidosis (Cox JW et al., Biochem Pharmacol 1989,38, 3535). The relationship between phospholipid metabolism and drug-induced toxicity remains enigmatic.
This unusual phospholipid has also been described in various obligatory and facultatively alkalophilic Bacillus strains (Clejan S et al., J Bacteriol 1986, 168, 334).
An alanyl derivative of bis(acylglycerol)phosphate was isolated from various bacteria and characterized (Fisher W et al., J Bacteriol 1998, 180, 2093).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire