
GLYCOLIPIDS BASED ON GLYCEROL
These lipids consist of a mono- or oligosaccharide moiety linked glycosidically to the hydroxyl group of glycerol which may be acylated (or alkylated) with one or two fatty acids. Furthermore, these glycolipids may be uncharged and, therefore often called neutral glycoglycerolipids, or may contain a sulfate or a phosphate group.
The last IUPAC recommendations for the nomenclature of glycolipids may be found on the IUPAC site.
According to their structure they may be classified into the following groups :
These compounds contain most frequently one or two sugars linked glycosidically to glycerol or diacylglycerol. Glycolipids with three or four sugars are also known. They are especially important in higher plants, algae and bacteria where they are located in photosynthetic membranes, they are also found in animals but in lesser amounts.
Photosynthetic membranes of all oxygenic photosynthetic organisms are constituted of about 85 % of neutral glycoglycerolipids (MGDG and DGDG) (Murata et al., Biochim Biophys Acta 1990, 1019, 261). Therefore, based on the natural abundance of photosynthetic organisms, these glycolipids constitute the most profuse lipid class on Earth (Gounaris K et al., Trends Biochem Sci 1983, 8, 378). The discovery of a plastid in Plasmodium falciparum, the causative agent of malaria, without galactoglycerolipids raised the question of the loss of galactoglycerolipids during evolution (Botté CY et al., Trends Plant Sci 2014, 19, 71).
Galactosyl monoacylglycerol with various numbers of galactose moiety has also been described in several plants.
Neutral glycoglycerolipids may be separated into two families :
1 – compounds with non acylated glycoside moiety
2 – compounds with acylated glycoside moiety
1 – Non acylated glycoside moiety
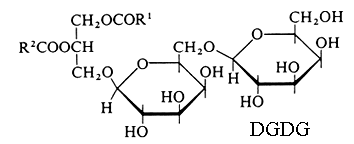
Monogalactosyl diacylglycerol (MGDG) and digalactosyl diacylglycerol (DGDG).
Glycoglycerolipids were unknown until 1956, when Carter HE et al. reported the presence of these lipids in lipid extracts of wheat flour (J Amer Chem Soc 1956, 78, 3735). Their specific association with photosynthetic tissues (tylakoid membranes of chloroplasts) was known from 1958 (Benson AA et al., J Am Chem Soc 1958, 80, 4740) but their correct structure was elucidated in 1961 (Carter HE et al., J Lipid Res 1961, 2, 215 and 223). This tight assocIation is exemplified by the herbicide or algaecide activity of a monogalactosyldiacylglycerol synthase inhibitor (Galvestine) (Bonneau AL et al., Patent EU 07290684.5).
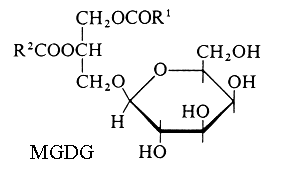
R1 and R2 are the two fatty chains
Higher homologues of galactolipids (tri- and tetragalactosyl diacylglycerols) were later identified in all plant tissues. A new glyceroglycolipid whose structure was elucidated to be trigalactosyl monolinolenylglycerol has been isolated from a Chinese folk medicine Premna microphylla (Zhan ZJ et al., Lipids 2003, 38, 1299).
Both MGDG and DGDG contain large amounts of linolenic acid (18:3n-3) and a specific trienoic acid (16:3 n-3). In higher plants, linolenic acid is almost the only fatty acid in MGDG, this composition led to name these plants "18:3 plants". In angiosperms, linolenic acid is concentrated in both sn-1 and -2 positions of both MGDG and DGDG and 16:3 n-3 is absent. By contrast, lower plants (green algae, mosses, ferns, conifers) and some angiosperms (Solanaceae, Brassicaceae, Chenopodiaceae) have 16:3 n-3 concentrated in the sn-2 position of galactolipids, while the low proportion of 18:3 n-3 is acylated in both positions. Such plants are called "16:3 plants" and have a "prokaryotic" structure similar to that observed in cyanobacteria. In red algae and photosynthetic diatoms, galactolipids are characterized by a high proportion of 20:5 n-3. Several n-3 fatty acids have been determined in MGDG isolated from the brown alga Sargassum thunbergii (Kim YH et al., Lipids 2007, 42, 395). Combinations of 18:3/20:5, 18:3/18:4, 18:4/20:5 and 18:3/18:3 were described.
It has been shown that MDA oxidatively generated from fatty acids in Arabidopsis may be recycled back into chloroplast 18:3-16:3-MGDG that may be again fragmented into MDA (Schmid-Siegert E et al., J Biol Chem 2016, 291, 13005).
Galactolipids have been found in only low amounts in most fungi, the main glycolipids being glycosphingolipids.
Another unusual fatty acid (18:3 n-1) was described to be abundant (about 25 %) in MGDG from marine diatom Skeletonema costatum (d’Ippolito G et al., Biochim Biophys Acta 2004, 1686, 100). These two fatty acids were shown to be metabolized into short-chain aldehydes, octadienal (8:2 n-4) and octatrienal (8:3 n-1), which may have deleterious effects on zooplankton crustaceans. A third aldehyde, heptadienal (7:2 n-3), was also shown to be produced from MGDG eicosapentaenoic acid.
A monogalactosyl diacylglycerol containing two linolenic acid (18:3 n-3) acyl groups has been described in fruits of rose hips (Rosa canina) and was shown to be an anti-inflammatory agent (inhibition of cell migration). This may be directly related to the clinically observed anti-arthritis properties of rose hip herbal remedies (Larsen E et al., J Nat Prod 2003, 66, 994).
Other studies reported that galactosyl diglycerides from various sources have antitumor-promoting (Shirahashi H et al., Chem Pharm Bull 1996, 44, 1404), oxygen scavenging (Nakata K , J Biochem 2000, 127, 731), and virus neutralizing (Nakata K et al., J Biochem 2000, 127, 191) activities. More recently, DGDG synthesized or isolated from Clinacanthus leaves from Thailand exhibited anti-herpes simplex virus activity (Janwitayanuchit W et al., Phytochemistry 2003, 64, 1253). MGDG extracted from the invasive brown alga Sargassum muticum was shown to exhibit anti-microfouling activity (inhibition of the growth of bacteria and fungi), thus protecting any surface immersed in sea water (Plouguerné E et al., Mar Biotechnol 2010, 12, 52).
There is evidence that specific galactolipids containing 16:3n-3 or 18:4n-3 in the sn-1 position and 20:5n-3 or 18:5n-3 in the sn-2 position may be directly involved in cytotoxic reactions induced by marine diatoms (Phaeodactylum tricornutum) (Andrianasolo EH et al., J Nat Prod 2008, 71, 1197). This is the first report of an apoptosis induction by galactolipids.
It has been discovered that the chloroplasts of Arabidopsis contained a particular MGDG named arabidopside characterized by the presence of 12-oxo-phytodienoic acid (Stelmach BA et al., J Biol Chem 2001, 276, 12832). This compound, a phytohormone closely related to jasmonic acid, is acylated on the sn-1 carbon of glycerol, the 16:3 n-3 being acylated in position sn-2. Later, other arabidopside species have been isolated with a glycerol acylated by two jasmonate compounds. It must be noticed that these glycolipids, considered as a kind of hormonal storage, seem to characterize only Brassicaceae. Curiously, the jasmonates remain in a free state in the chloroplasts of many other plants. MGDG containing 12/10-carbon oxoacids have been detected in disrupted leaves of Arabidopsis and have been related to the production of six-carbon aldehydes, alcohols and their esters which are known as leaf volatiles (Nakashima A et al., J Biol Chem 2013, 288, 26078).
A novel family of oxylipin-containing MGDG, named linolipins, has been discovered in the flax (Linum usitatissimum) leaves (Chechetkin et al., FEBS J 2009, 276, 4463). First members of this family were identified as MGDG species possessing one or two esterified residues of divinyl ether (w5Z)-etherolenic acid. Similar oxylipin-containing DGDG have been isolated from the leaves of flax plants inoculated with phytopathogenic bacteria or damaged by freezing-thawing (Chechetkin IR et al., Phytochemistry 2013, 96, 110).
It has been shown that the DGDG synthesis increased during flower development in Petunia hybrida, the pistils and pollinic tubes containing higher amounts of this glycolipid than other floral organs (Nakamura Y et al., Lipids 2003, 38, 1107).
In bacteria sn-3-O-glycosyldiacylglycerols may have as glycoside moiety: a-D-glucopyranoside (Pneumococcus, Staphylococcus), b-D-glucopyranosyl(1->6)-O-b-D-glucopyranoside (gentobiosyldiacylglycerol) (Staphylococcus), a-D-glucopyranosyl(1->2)-O-a-D-glucopyranoside (Mycoplasma), a-D-mannopyranosyl(1->3)-O-D-mannopyranoside (Micrococcus), b-D-galactofuranoside (Mycoplasma), or a-D-galactopyranosyl(1->2)-O-a-D-glucopyranoside (Lactobacillus).
A novel glycoglycerolipid containing an ether-linked alkyl chain in position C-3 of glycerol has been described in the bacteria Propionibacterium propionicum (Pasciak M et al., J Biol Chem 2003, 278, 3948). The glycoside moiety is a-D-glucopyranoside(1->3)a-D-glucopyranoside, glycosidically linked to the C-1 position of glycerol.
A diglucosyl diglyceride with a very rare a(1->4) diglucosyl structure has been isolated from the thermophilic bacterium Thermotoga maritima (Manca MC et al., Biochim Biophys Acta 1992, 1124, 249). Curiously, the glycerol moiety is esterified by two moles of palmitic acid. This glycolipid was found also acylated on the 6-OH of the terminal glucose by one molecule of decanoic acid.
It has been established that the glycolipids contained in flour act as surfactants and that they positively affect the volume, texture, and staling of bread. Therefore, this group of polar lipids can be regarded as a very interesting surfactant group for bread making. Monoalactosylglyceride and its lyso derivative were shown to be the most efficient compounds in improving the baking performance of wheat flour (Selmair PL et al., J Agric Food Chem 2008, 56, 6691). A review of the role of glycolipids in breadmaking may be consulted (Selmair PL et al., Lipid Technol 2010, 22, 7).
DGDG mono-estolides were reported in oat kernels by Hamberg M (Lipids 1998, 33, 355).
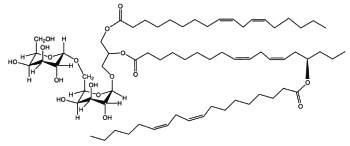
DGDG mono-estolide from oat kernel
That DGDG estolide contains a linoleic acid group and a 15-hydroxylated linoleic acid (avenolic acid). The last one is secondarily acylated by linoleic acid.
DGDG di-estolides have also been isolated from oat kernels (Moreau RA et al., Lipids 2008, 43, 533). Its basic structure is similar to the previous estolide but the third acylated lipid is not linoleic acid but avenolic acid, which is acylated by a normal linoleic acid moiety (seen Fig. below).
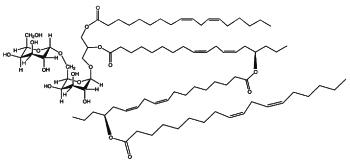
DGDG estolide from oat kernel
A small fraction of TriGDG and TetraGDG estolides was also detected. It could be determined that estolides comprise about 29% of the total glycolipid fraction and 10% of the total methanol-extractable lipid fraction.
An original glycoglycerolipid (2-Acetoxy-1-(3-glycosyloxyoctadecanoyl)glycerol) has been isolated from the exudates from glandular trichome-like secretory organs of Cerasu yedoensis, a commonly cultivated species of cherry tree in Japan (Asai T et al., Phytochem Lett 2011, 4, 38). The disaccharide moiety is linked to a 3-hydroxyoctadecanoic acid which esterifies glycerol in sn-1, the position sn-2 being acylated.
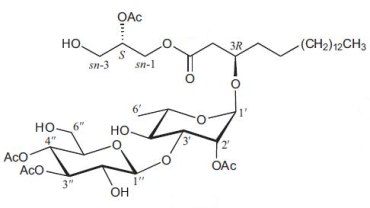
2-Acetoxy-1-(3-glycosyloxyoctadecanoyl)glycerol
Alkyl-acyl glycosylglycerols
An intermediate form of monoglycosyldiacylglycerol, cramerides, was isolated as a mixture of parent molecules from the sponge Pseudoceratina crassa (Costantino V et al., J Org Chem 1993, 58, 186). These compounds have a unusual cyclitol moiety, a branched alkyl chain and a saturated acyl chain with 14-16 carbon atoms, branched or not. One form is shown below.
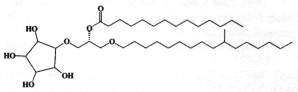
These compounds were shown to have antifeedant activity in fish, thus suggesting a role of feeding deterrents.
Many diether lipids from bacterial in origin which contained glycosidic headgroups were detected in carbonate chimney in a hydrothermal field (Bradley AS et al., Org Geochem 2009, 40, 1169). These compounds represent an unprecedented combination of archaeal characteristics in bacterial lipids since these lipids have a structure of glycosidic non-isoprenoid diethers.
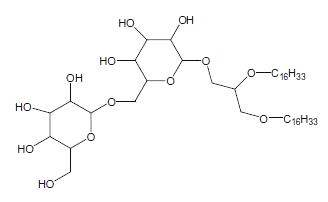
Diglycosyl alkylglycerol
The polar head group can be a mono or a diglycosyl moiety and the alkyl chains with 14-18 carbon atoms can be saturated, monounsaturated or monomethylated. The presence of these lipids is likely related to the very low level of phosphate in the vent environment. As these glycolipids were found in an environment analog to that which was present at the beginning of life on earth, it was speculated that glycolipids were an evolutionary predecessor to phospholipids.
Alkyl-diglycosylglycerols
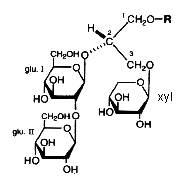
In the course of studies on bioactive substances from marine organisms, the discovery of a novel glyceroglycolipid was recently reported (Aoki S et al., Tetrahedron 1999, 55, 14865).
The chemical analysis revealed the presence of an O-alkyl ether chain (R = 16 carbon chain with or without a branched methyl group) at C1 of the glycerol, two moles of glucose at C2 and one mole of xylose at C3. These new lipids which have anti tumor properties were named Myrmekiosides (from the sponge Myrmekioderma sp).
An unique ether lipid characterized by the glycosylation (by xylose) of two alcohol radicals of glycerol and an O-alkenyl ether chain (24 carbon atoms and one double bond) at the third radical, was described in the Senegalese sponge Trikentrion loeve (Costantino V et al., Tetrahedron 1993, 49, 2711).
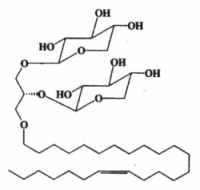
Another ether lipid, a plasmal conjugate to glycerol and psychosine, glyceroplasmalopsychosine, has been described in human brain.
Acylated forms of glycoglycerolipids were identified in extracts from a Cyanobacterium, Synechocystis sp (Kim YH et al., Lipids 1999, 34, 847). Their structural elucidation has shown that two forms were present, a palmitoyl group being esterified to the hydroxyl group at the C-6 position of the terminal glycosyl moiety of either digalactosyl monoacylglycerol (acylated DGMG) or digalactosyl diacylglycerol (acylated DGDG). The presence of acylated DGDG, MGDG and even monogalactosyl monoacylglycerol (MGMG) was reported from leaf homogenates (Heinz E et al., Hoppe-Seyler’s Z Physiol Chem 1969, 350, 493 and 1974, 355, 612) and nitrogen-fixing cyanobacteria (Murakami N et al., Chem Pharm Bull 1993, 41, 1177).
An unusual glucosamidyl glycolipids has been described in an extreme thermoacidophile Bacillus acidocaldarius (Langworthy TA et al., Biochim Biophys Acta 1976, 431, 550). This major compound, which comprises about 64% of the total lipids, appears to be a fatty N-acyl derivative of :
Glucopyranosyl(1->4)Glucosamine(1->3-diacylglycerol. The amide-linked fatty acid was primarily branched heptadecanoic, but also 11-cyclohexylundecanoic or 13-cyclohexyltridecanoic acid.
In bacteria and algae a large number of glycolipids containing different sugar combinations have been reported.
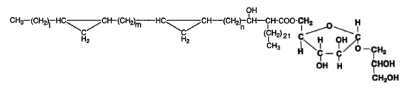
Mycoloyl arabinosylglycerols
These new glycolipids were isolated from a strain of the Mycobacterium avium-M. intracellulare complex (Watanabe M et al., J Bacteriol 1999, 181, 2293). These compounds were characterized by an arabinofuranosyl glycerol acylated on the carbon 5 of the arabinose moiety by one of various forms of mycolic acids (mycolic, keto mycolic, wax-ester mycolic). One form is shown below.
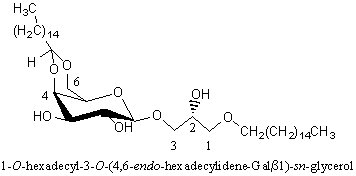
Alkyl-galactosylglycerols with acetal group
A novel galactosylalkylglycerol modified with a long-chain cyclic acetal at the sugar moiety was isolated from equine brain and named plasmalogalactosylalkylglycerol (Yachida Y et al., J Lipid Res 1999, 40, 2271). The chain lengths of alkyl and acetal groups were C14 for the former and C16 and C18 for the latter. The acetal group appears thus similar to that found in plasmalogalactosyl ceramide previously isolated from equine brain. The whole equine brain contains about 5 mg of this new glycoglycerolipid.
We include in that type all glycoglycerolipids containing at least one phosphate group whatever its position, either attached to one sugar or to one glycerol.
If we except phosphoinositides which contain one mole of inositol and could be classified into this group but are rather considered as "phospholipids", the glycophospholipids have been mainly described in bacteria. Some complex structures, the glycosyl phosphatidylinositol anchors, are found in all living creatures.
The simplest form of glycophospholipids are based on the simplest phospholipid, phosphatidic acid, linked to a glycosy group. The simplest of these compounds is glucosylated phosphatidic acid which was found in the red blood cells of the human umbilical cord (Nagatsuka Y et al., FEBS Lett 2001, 497, 141).
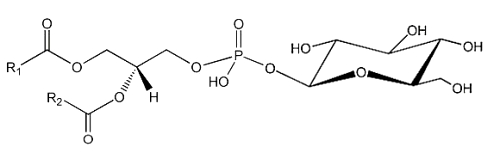
This phosphatidylglucoside was also identified in the rat brain, human neutrophils and several human epithelium cells. It could be also a new marker for lipid rafts (Nagatsuka Y et al., Biochim Biophys Acta 2008, 1780, 405). Curiously, this glycolipid is composed exclusively of a single pair of saturated fatty acids (18:0 and 20:0). Its function remains unknown.
It is well known that sugars and carbonyl compounds interact with amino acids or proteins in a sequence of reactions known as the Maillard reaction. Similarly, it has been shown that phosphatidylethanolamine also reacts with glucose leading, through an unstable Schiff base, to a phosphatidylethanolamine-linked Amadori product (Bucala R et al., Proc Natl Acad Sci USA 1993, 90, 6434).
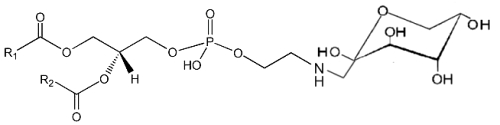
These compounds were detected in the rat liver and their concentration was increased in diabetic rats (Pamplona R et al., Life Sci 1995, 57, 873). Later, their correct structure was described in human red blood cells (Lertsiri S et al., Biosci Biotechnol Biochem 1998, 62, 893). Intensive researches have shown that Amadori-lipid products accelerate membrane lipid peroxidation in generating oxidative stress which alters cell integrity and survival (Oak JH et al., FEBS Lett 2000, 481, 26). Amadori-PE concentrations have been determined to be higher in plasma and red blood cells of diabetic patients when compared to healthy subjects (Shoji N et al., J Lipid Res 2010, 51, 2445).
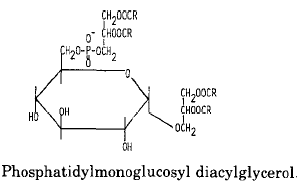
An example of glycophospholipids found in many Gram-positive bacteria is phosphatidyl monoglucosyl diacylglycerol whose structure and metabolism were elucidated in Pseudomonas diminuta (Shaw JM et al., J Biol Chem 1977, 252, 4395).
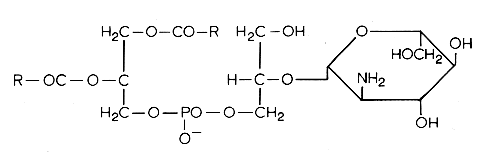
Another example of these lipids is a phosphatidyl glucosaminyl glycerol found in Bacillus megaterium
Another phosphoglycolipid has been isolated from several species of the halophilic Gram-negative bacteria Halomonas (Giordano A et al., J Lipid Res 2007, 48, 1825). The structure was established to be a phosphatidic acid linked to a glycerol residue (phosphatidylglycerol moiety) which is alkylated by a glucose group. The most abundant acyl chains linked to the phosphatidylglycerol moiety are palmitic and oleic acids and C16:0, C19:cyclopropane.
A derivative of N-acetylglucosaminyl diacylglycerol in which a phosphoethanolamine unit is attached to the 6′-position of the sugar has been isolated from Clostridium tetani, the causative agent of tetanus (Johnston NC et al., J Lipid Res 2010, 51, 1953).
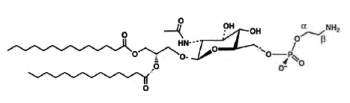
N-Acetylglucosaminyl-phosphoethanolamine diacylglycerol
Another example is phosphatidylinositol polymannoside found in Mycobacteria
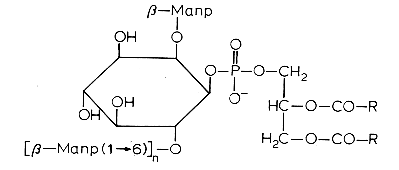
in position 2′ of the inositol there is a mannopyranoside group and in position 6′ there may be one sugar (dimannoside), three (tetramannoside), four (pentamannoside), or five (hexamannoside) sugar groups.
It was found that phosphatidylinositol mannosides from Mycobacterium tuberculosis exert potent anti-inflammatory effects in a lung model in vitro and in vivo via an inhibition of Toll-like receptor and inhibition of the production of NO, cytokines and chemokines (Doz E et al., J Biol Chem 2009, 284, 23187). These effects may be a strategy developed by mycobacteria for repressing the host innate immunity. Analogues are in development for clinical essays.
The presence of the mannophosphoinositides is a striking feature of the phospholipid composition of the Actinomycetes and of some bacteria. Monomannosides have been reported in some Streptomyces, Mycobacterium species and in Propionibacteria. Two types of dimannosides, differing in their fatty acid were identified in Streptomyces griseus. They have at least one additional fatty acid attached to a mannose residue (tri- and tetra-acylmannoside). The most complex polymannosides were identified in Mycobacteria, sometimes with several fatty acids acylating the mannose chain.
Acylated phosphatidylinositol dimannosides were also described in Corynebacterium urealyticum, a C16:0 or C18:1(R3) acylating one of the two mannose moieties (Yagüe G et al., Microbiology 2003, 149, 1675).
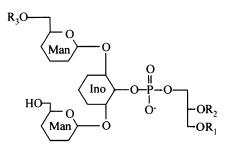
It has been shown than phosphatidylinositol dimannoside was the "anchoring domain" of the lipopolysaccharides of mycobacteria, lipoarabinomannan and lipomannan, of key importance in host-pathogen interaction (Hunter SW et al., J Biol Chem 1990, 265, 9272). This structure was the first demonstration of a prokaryotic version of the phosphatidylinositolglycans which are well known in anchoring cell-surface proteins (Ferguson MAJ et al., Annu Rev Biochem 1988, 57, 285). The complete structural features of lipoarabinomannan from Mycobacterium bovis, largely used around the world as vaccine against tuberculosis, have been described (Venisse A et al., J Biol Chem 1993, 268, 12401).
A review of the distribution and composition of these glycophospholipids and of other Actinomycetes lipids may be consulted with interest (Verma JN et al., Adv Lipid Res 1983, 20, 257).
Lipophosphoglycan is the predominant glycolipid present at the surface of parasitic protozoa such as Leishmania, Entamoaba and Crithidia (review in: Guha-Niyogi A et al., Glycobiology 2001, 11, 45). It is composed of four domains, (1) an alkyl lyso phosphatidylinositol anchor, (2) a glycan core, (3) Gal-Man-PO4 backbone repeated units, and (4) an oligosaccharide cap structure (Descoteaux A et al., Biochim Biophys Acta 1999, 1455, 341). Among species, the lipid anchor, the glycan core and the Gal-Man-PO4 backbone are completely preserved while variations are found in the carbohydrate chain and in the cap structure (Mc Conville MAJ et al., Biochem J 1995, 310, 807). Lipophosphoglycan has been implicated in a number of functions within the mammalian host.
Glycosylated cardiolipin was first detected in several strains of Streptococcus group B and identified as glucopyranosyl cardiolipin (Fisher W, Biochim Biophys Acta 1977, 487, 74).
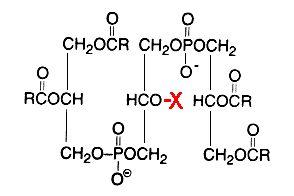
X = glucosyl residue
This derivative amounts to about 18% of the lipid phosphorus in Streptococcus but is only a minor component in Vagococcus fluviatilis (Fisher W et al., J Bacteriol 1998, 180, 2950). This glycolipid is acylated mainly by palmitic acid (about 33%) and oleic acid (about 25%) (Fisher W, Biochim Biophys Acta 1977, 487, 89). This uncommon glycolipid was also found (4 mol% of total phospholipids) in a thermophilic bacteria Geobacillus steathermophilus where its fatty acid pattern exhibits predominantly iso-C15:0 and anteiso-C17:0 (Schäffer C et al., J Bacteriol 2002, 184, 6709). It was hypothesized that this compound could be involved in the regulation of the membrane lipid composition of G. stearothermophilus to compensate for the destabilizing effect of high temperatures on the membrane organization.
Several cardiolipin analogues of extremely halophilic prokaryotes belonging to the Archaea domain (Halobacterium salinarum) have been described (Corcelli A, Biochim Biophys Acta 2009, 1788, 2101).
A unique glycophospholipid present at a level of 33% of the total lipids has been identified in a Gram-positive bacteria Deinococcus radiodurans (Anderson R, Biochim Biophys Acta 1983, 753, 266).
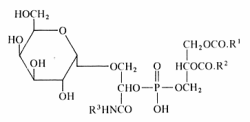
This lipid was described as the 3-(galactopyranosyl)-2-(3-phosphatidyl) glyceroyl derivative of a fatty alkylamine. The alkylamines (R3) are mainly straight-chain compounds, saturated (C15 to C17) or monoenoic (C16 to C18), with isomeric 17:1 bases preponderant.
Mycoplasma fermentans was shown to produce a phosphocholine-containing glycoglycerolipid which has the phosphocholine group attached to the C6 of glucose (Matsuda K et al., J Biol Chem 1994, 269, 33123).
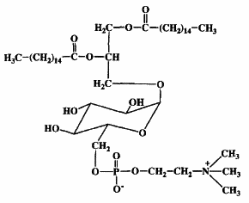
It has been hypothesized that this lipid might be involved in the pathogenesis of some diseases, as rheumatoid arthritis, through triggering of inflammation or cell death caused by their phosphocholine residue.
The chemical structure of another choline-containing phosphoglycolipids with two phosphate residues was described in M. fermentans. The major phosphoglycolipid (MfGL-II), which accounts for about 30% of the total polar lipid fraction, was identified as 6′-O-[3"-phosphocholine-2"-amino-1"-phospho-1",3" -propanediol]-alpha-D-glucopyranosyl-(1′-3)-1,2-diacyl-glycerol (Zahringer et al., J Biol Chem 1997, 272, 26262).
In the Gram-positive bacteria Lactococcus and Streptococcus, several complex glycolipids deriving from the kojibiosyl diacylglycerol structure (glucopyranosyl-1->2-glucopyranoside-diacylglycerol) were identified (O’Leary WM et al., in "Microbial lipids" vol 1, Ratledge C et al. eds, Acad Press, 1988).
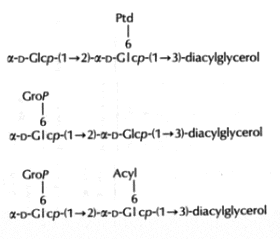
(Ptd: 3-phosphatidyl, Grop: glycero-1-phospho, Acyl: fatty acyl group)
Typically, these structures are derived by substitution at one or both of the 6-positions on glucose by a 3-phosphatidyl group, a glycerol-1-phospho group, or a fatty acyl group. In more complex cases, additional glycerophospho residues (and glycosyl and alanyl substituents) are incorporated (Fischer W in "Chemistry and biological activities of bacterial surface amphiphiles", Shockman GD et al., eds, Acad. Press 1981).
In membranes of Gram-positive bacteria (ex. Staphylococcus) more complex phosphorylated compounds can be found: lipoteichoic acids. They consist of polymer of glycerol-1-phosphate linked to a glycosyl diglyceride or a phosphatidyl glycosyl diglyceride. Some other substitutions on the glycerophosphate units with glycosyl or alanyl groups are also described (Lambert et al., Biochim Biophys Acta 1977, 472, 1).
The lipoteichoic acid found in Bacillus subtilis is illustrated below :
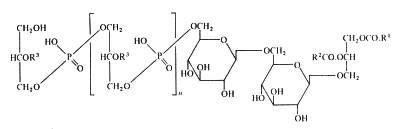
R1 et R2 are fatty acyl groups and R3 is mainly H, alanyl or acetylglucosaminyl
SULFOGLYCOGLYCEROLIPIDS
Lipids bearing a sulfur atom are an interesting group since they are said to be found in acidic membranes, i.e., membranes with a surface at a strongly acidic pH (about 2). Under these conditions carboxylic acids are in their un-ionized forms, but the sulfolipids with sulfate or sulfonic acid groups exist at least partially in the anionic form. Sulfolipids are found in fungi, algae, bacteria, the chloroplast of higher plants, but also in some mammalian cells.
A new type of sulfolipid was discovered in 1959 in the microalga Chlorella at the Scripps Institute of Oceanography (San Diego, Ca, USA) (Benson AA et al., Proc Natl Acad Sci USA 1959, 45, 1582). That lipid, sulfoquinovosyl diacylglycerol (SQDG), was shown to be derived from MGDG but with a sulfonic acid linkage on the galactosyl moiety. It was shown to be present in the thylakoid membranes of all lower and higher plants. Thus, photosynthetic membranes of plants, algae, cyanobacteria, and some fungi among the Basidiomycetes (Coprinus, Psalliota, Clitocybe) contain large amounts of that sulfolipid.
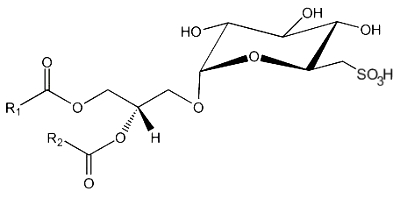
This lipid is the only lipid with a sulfonic acid linkage. It consists of a monoglycosyldiacylglycerol with a sulfonic acid in position 6 of the monosaccharide moiety. The sulfonoglucosidic moiety (6-desoxy-6-sulfono-glucoside) is described by the adjective sulfoquinovosyl (quinovose : 6-deoxy-D-glucose). R1 is frequently palmitic acid, R2 being the unsaturated chain (most often 16:3n-3).
A review summarizes recent research defining the mechanism for SQDG biosynthesis and its biological function in higher plants, particularly under phosphate-starved conditions (Shimojima M, Prog Lipid Res 2011, 50, 234).
An extract of the marine chloromonad Heterosigma carterae (Raphidophyceae) was shown to contain a complex mixture of sulfoquinovosyl diacylglycerols (Keusgen M et al., Lipids, 1997, 32, 1101). The main fatty acyl groups consisted of 16:0, 16:1(n-7), 16:1(n-5), 16:1(n-3) and 20:5 (n-3). Sulfoglycoglycerolipids were described in the green algae Elakatothrix and Zygnema and in some Phaeophyta (Fucus, Pelvetia).
Occurrence of SQDG has been demonstrated in the symbiotic bacteria Rhizobium meliloti as well as in other strains of symbiotic Rhizobiaceae (Cedergren RA et al., J Lipid Res 1994, 35, 1452). Taking into account this first report of the presence of SQDG outside the plant kingdom, photosynthetic bacteria or diatoms, a role for that sulfolipid may be suggested in the Rhizobium/legume relationship. Some sulfate esters are also found in halophilic bacteria (Halobacterium), but they have two alkyl diphytanylether chains (Haines TH, in CRC handbook of microbiology, vol. 5, CRC Press 1979).
SQDG forms were also extracted from a sea urchin (Scaphechinus mirabilis) (Logvinov SV et al., Chem Nat Comp 2012, 48, 175). The carbon chains included saturated (14:0 to 24:0) and mono-unsaturated fatty acids (20:1 to 24:1).
Curiously, an anti HIV-1activity was reported for SQDG from cyanobacteria containing palmitic and linolenic acids (Gustafson KR et al., J Natl Cancer Inst 1989, 81, 1254).
In several mammalian tissues (brain, spermatozoa) sulfate esters of galactoglycerolipids are found. The acyl groups R’ and R” are most frequently saturated (16 or 18 carbon atoms). Kornblatt MJ et al. (Biochem Biophys Res Commun 1972, 48, 1489) discovered these lipids in the testes of several mammalian species.
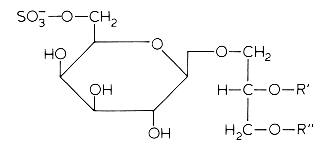
The concentrations of these sulfated glycolipids in testis and spermatozoa are about 0.3-1 mmole/g wet tissue (3% of total lipids and more than 90% of total glycolipids in mammalian testis).
In brain and in testis, the sulfoglycoglycerolipids occur as a mixture of alkylacyl (seminolipids) and diacyl glycerol. These compounds could be associated in the nervous system with the process of myelination (Pieringer J et al., 1977, 166, 421). Evidence was provided that seminolipids are essential for normal spermatogenesis in mice (Fujimoto H et al., J Biol Chem 2000, 275, 22623).
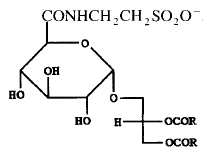
A tauroglycolipid has been isolated from a seawater bacterium Hyphomonas jannaschiana which is devoid of phospholipids (Batrakov SG et al., Biochim Biophys Acta 1996, 1302, 167). The structure of that lipid, shown below, was determined to be 1,2-diacyl-3-glucuronopyranosyl-glycerol taurine-amide. Its main fatty acyl chains are 16:0, 16:1n-7, 18:0, 18:1n-7 and 19:0.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire