
The hydroxyl group(s) may occur at various positions in the carbon chain which can be saturated or monoenoic.
Some polyhydroxy fatty acids are also known, which are most frequently produced by lipoxygenase activities, as for several mono-hydroxylated fatty acids.
In some bacteria, complex hydroxy, branched-chain fatty acids (mycolic acids) are described.
MONOHYDROXY FATTY ACIDS
a-Hydroxy acids or 2-hydroxy acids are found in plants (chain from 12 up to 24 carbon atoms) and in animal wool waxes, skin lipids and specialized tissues, mainly in brain.
2-Hydroxylinolenic acid was reported at a level of 13% in the seed oil of the Labiateae Thymus vulgaris (Smith CR et al., Lipids 1969, 4, 9). Along with the previous one, 2-hydroxylinoleic and 2-hydroxyoleic were detected in another Labiateae Salvia nilotica (Bohannon MB et al., Lipids, 1975, 10, 703). Several 2-hydroxy fatty acids have been reported to be present in polar lipids of the alga Grateloupia turuturu from Britany, France (Kendel M et al., Lipids 2013, 48, 535).
2-Hydroxytetracosanoic acid (cerebronic acid) and 2-hydroxy-15-tetracosenoic acid (hydroxynervonic acid) are constituents of the ceramide part of cerebrosides (glycosphingolipides found mainly in nervous tissue and in little amount in plants).
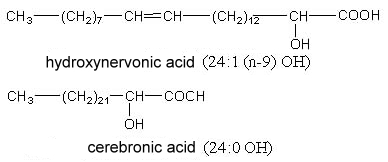
The testis and spermatozoa of boar and rat contain sphingomyelin with 2-hydroxylated n-6 tetra- and pentaenoic acids with very long carbon chain (up to 34 carbon atoms) (Robinson BS et al., J Biol Chem 1992, 267, 1746). It has been postulated that these lipids play a role in reproduction.
Several studies have uncovered potent biological activities of 2-hydroxyoleic acid (Minerval). This synthetic derivative of oleic acid was found to have potent anti-cancer activities in vitro and in animal models (Martinez J et al., Mol Pharmacol 2005, 67, 531; Llado V et al., J Cell Mol Med 2010, 14, 659). The anti-cancer activity of 2-hydroxyoleic acid is mediated, at least in part, by downregulation of dihydrofolate reductase (Llado V et al., PNAS 2009, 106, 13754). The mechanism of its action is not fully understood, and it has been attributed to its structural effects on cell membranes, rather than specific interactions with target proteins. Nevertheless, it seems that the pro-apoptotic activity of 2-hydroxyoleic acid explains the effectiveness of this non-toxic anticancer drug (Llado V et al., J Cell Mol Med 2010, 14, 659). New results have shown that this fatty acid is able to regulate the synthesis of sphingomyelin in glioma cells in restoring normal membrane levels and triggering cell cycle arrest (Barcel-Coblijn G et al., PNAS 2011, 108, 19569).
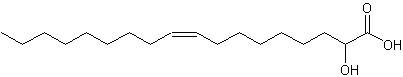
2-Hydroxy-9-cis-octadecenoic acid
The addition of 2-hydroxy palmitic acid to different cell lines increased their sensitivity to the synthetic antitumor drug, PM02734 (Herrero AB et al., Cancer Res 2008, 68, 9779).
A review of fatty acid 2-hydroxylation in sphingolipid biology in connection with the nervous system and various cell types may be consulted (Hama H, Biochim Biophys Acta 2010, 1801, 405). Curiously, among the enantiomers, the (R)-enantiomer is enriched in hexosylceramide whereas the (S)-enantiomer is preferentially incorporated into ceramide (Guo L et al., J Lipid Res 2012, 54, 1327).
The analysis of long-chain hydroxylated fatty acids in ancient ceramics or shells enables to identify the origin of these deposits. Thus, 13,14-dihydroxydocosanoic acid and 11,12-dihydroxyeicosanoic acid, derived from erucic acid (C22:1) and gondoic acid (C20:1) were shown to be biomarkers for seed oil from Brassicaceae plants such as rapeseed (Brassica napus) or radish (Raphanus sativus) (Romanus K et al., Anal Bioanal Chem 2008, 390, 783). Goni and Hedges (1990b) showed
Leaves of angiosperms and different gymnosperm plant families could be distinguished by the abundance of 14-hydroxytetradecanoic acid and positional isomers of dihydroxyhexadecanoic acid (Goni MA et al., Geochim Cosmochim Acta 1990, 54, 3073). The hydroxylated fatty acids from leaf-derived lipids have been proposed as plant biomarkers in soils and sediments (Mueller KE et al., Org Geochem 2012, 52, 130).
Polyhydroxyalkanoates : The chemical composition of lipid inclusions in Bacillus megaterium was identifed in 1926 as poly(3-hydroxybutyric acid) (Lemoigne M, Bull Soc Chim Biol 1926, 8, 770). Since that discovery, a large variety of bacteria were shown to synthesize polyesters (polyhydroxyalkanoates) forming linear chains of esterified 3-hydroxy acids (Kim YP et al., Adv Biochem Eng Biotechnol 2001, 71, 51). More than 90 different monomer units have been identified as constituents of polyhydroxyalkanoates in various bacteria. A few members include poly(3-hydroxybutyrate) or poly(3-hydroxybutyrate-co-3-hydroxyvalerate. They are sometimes, but erroneously, considered to be a carbohydrate, but their solubility characteristics are those of a lipid, except for high molecular weight homopolymers of 3-hydroxybutyrate units which are acetone-insoluble.
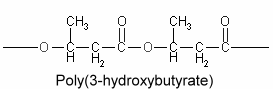
Poly(3-hydroxybutyrate) is the most widespread and best characterized lipid polymer. These polyesters accumulate as intracellular inclusions and act as a carbon and energy reserve. They are produced by some bacteria when they have extra energy, then used as an energy source when needed. They have a great industrial interest because of their plastic and elastomer properties and as a source of biodegradable polymers for low-value commodity products. Their synthesis in crop plants would allow an efficient large-scale production which may lead to new substitutes for petroleum-derived plastics (Rezzonico E et al., Phytochemistry Rev 2002, 1, 87). Genetic engineering has been done to transfer the polymer-making capacity to Escherischia coli and to higher plants. It is theoretically possible to modify starch forming plants (such as potatoes) to grow these polymers.
Several lipid-like fractions (acetone-soluble) are copolymers containing both short- and medium-chain-length 3-hydroxyalkanoate units. They have been identified as various hydroxyalkanoic acids with 3 to 14 carbon atoms (Steinbuchel A, 1991, Polyhydroxyalkanoic acids. In: Byrom D (ed) Biomaterials. Macmillan, New York, p 123). Two groups of bacteria have been described : those which produce short-chain polyhydroxyalkanoates (C3-C5 monomer units) and those producing medium-chain polyhydroxyalkanoates (C6-C14 monomer units). Some bacteria (Rhodospirillum, Rhodocyclus, Rhodococcus, Aeromonas, Pseudomonas) have been shown to accumulate polyesters containing short- and medium-chain-length 3-hydroxyalkanoic acids. A copolymer with a molecular mass of about of 40 000 containing 3-hydroxybutyric acid (3HB) and several other 3-hydroxyalkanoic acids has been described in Pseudomonas sp (Kato M et al., Appl Microbiol Biotechnol 1996, 45, 363). A part of the described structure is shown below.
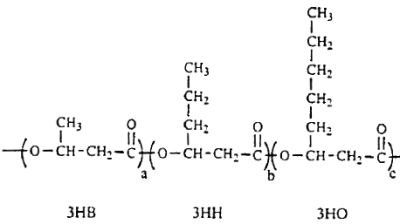
A fragment of polyhydroxyalkanoate containing 3HB (3-hydroxybutyrate), 3HH (3-hydroxyhexanoate) and 3HO (3-hydroxyoctanoate) units
Applications are being developed to produce and extract these bacterial polymers for use in industry, including molded goods, paper coatings, non-woven fabrics, adhesives, films, and polymer additives. The biocompatible nature of polyhydroxyalkanoates and their potential applications in the medical field should also not be overlooked. Biodegradable plastics and polymers are certain to increase in importance as environmental contamination and waste disposal problems associated with synthetic plastics become more severe. Metabolix Inc. of Cambridge, Mass USA, has taken up the challenge to further develop efficient technologies for polyhydroxyalkanoates production (http://www.metabolix.com/index.html).
b-Hydroxy acids or 3-hydroxy acids occur in some bacterial lipids. 3-Hydroxy oxylipins are widely distributed in nature, occurring in mammals, bacteria and yeasts. In mammalian systems, production of 3-hydroxy oxylipins is mainly attributed to fatty acid oxidation disorders.
Since 3-hydroxy fatty acids are unique structural components of the endotoxin (LPS), characteristic of Gram-negative bacteria, they can be employed as biomarkers for estimating the amount of these bacteria in atmospheric aerosols (Lee AKY et al., Atm Envir 2004, 38, 6307). Nevertheless, there are limitations in their use when analyzing animal tissues since they are also produced by mitochondrial beta-oxidation (Szponar B et al., J Microbiol Meth 2002, 50, 283). Chemically, endotoxin is lipopolysaccharides which is a major constituent of the outer membrane of Gram-negative bacteria. The lipid portion of the endotoxin, lipid A, is chemically distinct from all other lipids in biological membranes and consists of characteristic 3-hydroxy fatty acids, primarily with carbon chain lengths from 10 to 18, attached to hydroxyl and amino groups of a disaccharide backbone. It has been proposed that 3-hydroxy fatty acid quantification may be employed as biomarkers of endotoxins and Gram-negative bacterial community in atmospheric aerosols (Lee AKY et al., Atmosph Environ 2004, 38, 6307). This approach is more iformative than the determination of mass loading, total endotoxin concentration in aerosols.
The LPS layer is not limited to bacteria. The presence of this cell wall component has been reported in a medically important higher basidiomycete, Antrodia camphorata (Cheng J. et al., J Agric Food Chem 2005, 53, 469), this fungal LPS reversing the immuno-regulating properties exerted by bacterial LPS.
It must be noticed that 3-hydroxypalmitic acid methyl ester has been shown to be a very potent autoregulator compound controlling virulence in the phytopathogenic bacterium Ralstonia solanacearum (Flavier AB et al., Mol Bacteriol 1997, 26, 251). This fatty acid methyl ester was shown to be an intercellular signal active via a volatile phase.

3-Hydroxypalmitic acid methyl ester
It was show that 3-OH oxylipins can effect quorum sensing in Candida albicans (Nigam S et al., Curr Microbiol 2010, 62, 55), a function used by microorganisms to measure population density and to regulate pathogenicity. Thus, this yeast utilises 3-OH oxylipins, i.e. 3-OH-14:2 produced from 18:2, as a signal for expression of genes responsible for accelerating cell morphogenesis at a certain population density.
It was also found that this yeast converts arachidonic acid, released from infected host cells, to a 3-OH oxylipin (3-hydroxy eicosatetraenoic acid or 3-HETE) via incomplete mitochondrial beta-oxidation. This compound then acts as substrate for the host cyclooxygenase-2 (COX- 2), leading to the production of potent pro-inflammatory 3-OH prostaglandin E2 (Ciccoli R et al., Biochem J 2005, 390, 737).
3-Hydroxy fatty acids with two conjugated double bonds and 16 or 18 carbon atoms have been described in Fijian green macroalgae (Tydemania expeditionis) (Jiang RW et al., Phytochemistry 2008, 69, 2495). These compounds demonstrated moderate inhibitory activity against a panel of tumor cell lines (IC50 : 1.3 to 14.4 mM). Three 3-hydroxy fatty acids mainly in the phospholipids have been described in polar lipids of the alga Grateloupia turuturu (Kendel M et al., Lipids 2013, 48, 535).
w-Hydroxy acids have their hydroxyl group at the methyl end of the carbon chain and can result in special glycerides with more than three acyl groups through acylation of one or more hydroxyl groups (ergot, Lesquerella and kamala oils). They participate also in the structure of suberin, a lipid polyester present in plant cell walls, and of cutin, a lipid polyester which is a component of the plant cuticle. These apoplastic structures are important plant-environment interfaces which act as barriers limiting water and nutrient loss and protecting plants from radiation and pathogens. After experimental depolymerization, monomers and oligomers containing glycerol are esterified by several hydroacids. The most frequent w-hydroxy acids were found to be C16, C:18, C18:1, C18:2 in cutin and C18 to C24 in suberin (Graca J et al., Phytochemistry 2002, 61, 205; Graca J et al., Chem Phys Lipids 2006, 144, 96; Pollard M et al., Tr Plant Sci 2008, 13, 236). In lipid polyesters extracted from Arabidopsis and Brassica seeds, w-hydroxy acids with 16 up to 26 carbon atoms were described (Molina I et al., Phytochemistry 2006, 67, 2597).
w-Hydroxy acids are the main building blocks of algaenan, the highly cross-linked constituent of the cell walls of green algae (Blokker P et al., Phytochemistry 1998, 49, 691). These ester-bound fatty acids are unsaturated, the monoene (n-9) having a C30, C32 or C34 carbon chain and the diene (n-18 and n-19) having a C30 and C32 carbon chain.
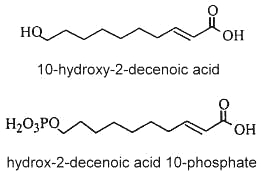
w-hydroxy decenoic acids occur in honey in small concentrations but are characteristic of the royal jelly of nurse bees (Apis mellifera). trans 10-Hydroxy-2-decenoic acid is considered as the genuine fatty compound of royal jelly (about 50 % of the total fatty acids) (Bloodworth BC, J AOAC Int 1995, 78, 1019), other hydroxylated fatty acids (10-hydroxydecanoic acid and 9-10-hydroxy-2-decenoic acid, 3,10-dihydroxydecanoic acid, 8-hydroxyoctanoic acid) were also detected. These pheromone components which are excreted in the salivary glands of bees are said to give specific therapeutic properties to royal jelly such as skin protection, bactericide, anti-inflammatory action, immuno-regulation and anti-cancer activities. Furthermore, they could be potential candidates in the treatment of atherosclerosis (Makino J et al., J Nat Prod 2016, 79, 1137). It has been shown that worker bees secrete acids functionalized at the last (w) position, such as 10-hydroxy-2-decenoic acid and its saturated counterpart, while the honeybee queen produces pheromones such as 9-hydroxy-2-decenoic acid, and other acids functionalized at the penultimate (w-1) position (Plettner E et al., Science 1996, 271, 1851).
Several parent molecules have been described in the royal jelly (Noda N et al., Lipids 2005, 40, 833). It has been found that these hydroxy fatty acids strongly activate the transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1) (Terada Y et a., J Agric Food Chem 2011, 59, 2627). These properties could be related to the observed effects of royal jelly on several pathologies.
Besides these compounds, other fatty acids were observed : a diacid, and mono- and diesters of 10-hydroxy-2-decenoic acid in which the hydroxyl group is esterified by another fatty acid unit (estolide-like molecules). In addition, a phosphorylated derivative was also detected (2E-decenoic acid 10-phosphate).
Different methods have been described for determination of 10-HDA in royal jelly, including HPLC and gas chromatography. The report of a study using HPLC and UV detection after ultrasound-assisted extraction may be consulted for literature review and technical aspects (Zhou J et al., Chromatographia 2007, 66, 185).
Due to their bifunctional nature, w-hydroxy fatty acids are used in various industrial products ranging from pharmaceuticals, cosmetics, coatings, surfactants and general polymer building blocks (Metzger JO et al., Appl Microbiol Biotechnol 2006, 71, 13). The biological production of w-hydroxy fatty acids represents an emerging biotechnology (Bitto NJ et al., Lipid Technol 2009, 21, 216).
w-Hydroxy octadecenoic acid : kamlolenic acid with the following structure, 18-hydroxy, 9c, 11t, 13t-18:3, is found in kamala oil, a product extracted from the seeds of Kamala tree (Mallotus phillipinensis, Euphorbiaceae) (Calderwood RC et al., J Sci Food Agric 1954, 5, 382). The kamala oil can be used as a substitute for tung oil, obtained from Aleurites spp., in the production of rapid-drying paints and varnishes. The seed oil is also used as a fixative in cosmetic preparations. The oil is also used as a fixative in cosmetic preparations and for coloring foodstuffs and beverages.
A large array of hydroxylated fatty acids deriving from linoleic acid, a-linolenic acid, roughanic acid (16:3 n-3), and other C20 fatty acids (arachidonic acid, EPA), mainly under the action of lipoxygenase, are named Oxylipins (Mosblech A et al., Plant Physiol Biochem 2009, 47, 511). The previously formed hydroperoxides are subsequently transformed by the action of different enzymes into these hydroxylated fatty acids but also into oxo fatty acids, divinyl ethers, volatile aldehydes, and jasmonates. They are widespread in nature occurring in plants, mosses, algae, bacteria, fungi and sometimes in animals. In general, oxylipins are bioactive metabolites involved in regulating developmental processes and in environmental and pathological responses.
Very-long-chain fatty acids (C28C34) containing a hydroxy group at the n-18 position have been identified in the microalgae from the genus Nannochloropsis (Gelin F et al., Phytochemistry, 1997, 45, 641). That constant position likely indicates that the series results from chain-elongation of a particular hydroxy fatty acid. Several hydroxylated fatty acids with 16 and 18 carbon atoms have been described in microalgae, the most abundant being hydroxylated on the C11, C8, and C13 (de los Reyes C et al., Phytochemistry 2014, 102, 152). These compounds are frequently inhibitors of the TNF-a production. Thus, the most active oxylipin was a C-16 hydroxy acid, which at 25 mM caused a 60% decrease of the TNF-a level in LPS-stimulated macrophages.
An oxylipin, 15-hydroxylinoleate, has been isolated from seeds of oat (Avena sativa) and was named avenoleic acid (Hamberg M et al., Phytochemistry 1996, 42, 729). That fatty acid seems specific for oat, as it was not detectable in seeds of barley, rye, or wheat. Further studies have shown that avenoleic acid was found to be mainly localized in the glycolipid fraction of oat seed lipids (Hamberg M et al., Lipids 1998, 33, 355).
Among the 12-hydroxy acids, the most abundant is ricinoleic acid (12-hydroxy-9-octadecenoic acid) which characterizes castor oil (from Ricinus communis). It was discovered in 1848 (Saalmüller L, Ann 1848, 64, 108). Goldsobel AG (Ber 1894, 27, 3121) showed that ricinoleic acid has the actual molecular structure. This acid is the only one hydroxylated fatty acid used in oleochemical industry. The seed oils of Jatropha gossypifolia and Hevea brasiliensis (Euphorbiaceae living in South America and India) were found to contain high content of ricinoleic acid (about 18%).
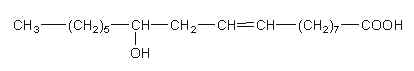
Ricinoleic acid is abundant in castor oil (90%) but many common vegetable oils and oil seeds contain lower amounts of that particular fatty acid. Thus, its content amounts to 0.27% in cottonseed oil, 0.03% in soybean oil, and 0.02% (Yamamoto K et al., Lipids 2008, 43, 457).
While known chiefly as a purgative, few decades ago, this fatty acid affords now a wide range of reactions enabling the formation of several derivatives. These chemicals are on a par with petrochemical products for use in several industrial applications. Castor oil and its derivatives are used in food (additive), textile (surfactants, pigment wetting agents), paper (defoamer, water proofing additive), plastics (Nylon-11, polyamide resin known as Rilsan 11 used for coating metals, plasticizers, coupling agents, tubes, films), perfumes and cosmetics (emulsifiers, deodorant), electronics (capacitor fluids, polyurethane and polyamide resins), pharmaceuticals, paints, inks, adhesives and lubricants. Castor oil is also used to make emulsifier after transesterification of fatty acids from the glycerol to the hydroxyl group in ricinoleic acid and ethoxylation to give castor oil polyethylene glycol (Diehl B, Lipid technol 2011, 23, 278).
The production of conjugated linoleic acid by dehydration and isomerization of ricinoleic acid has been described (Villeneuve P et al., JAOCS 2005, 82, 261).
Castor oil can be reacted with sulfuric acid to make Turkey-Red Oil, the first synthetic detergent or surfactant after ordinary soap, a predecessor to sodium lauryl sulfate. These properties are the result of the sulfonation of ricinoleic acid. Turkey-Red Oil is also used in the dyeing of cotton texture.
The limited amount of castor oil as natural source of ricinoleic acid has led chemists to develop suitable processes for the preparation of hydroxy fatty acids from commercial plant oils (Dahlke B et al., JAOCS 1995, 72, 349). The microbial transformation of ricinoleic acid with the yeast Yarrowia lipolytica yields g-decalactone, an aroma compound with fruity and oily notes found naturally in fruits and fermented foods ( Schrader J. et al., Biotechnol Lett 2004, 26, 463).
A fatty acid isomeric with ricinoleic acid, 9-hydroxy-12c-octadecenoic acid, has been shown to be of general occurrence (between 9 and 15%) in the seed oils of the genus Strophantus (Apocynaceae) (Gunstone FD et al., J Sci Food Agric 1959, 10, 522) but was also found in Holarrhena, Nerium and Wrightia of the same family. It was reported for the first time in the seed oil of the desert rose Adenium obesum in which it is present at a level of around 26 % (Smith MA et al., JAOCS 2016, 93, 105).
This fatty acid was named strophantus acid and it is a potential renewable feedstock for the oleochemical industry, mainly as a precursor for the synthesis of antimicrobial compounds.
As castor seed production presents some problems (toxicity of the seed, allergic reactions), Lesquerella species were proposed as a valuable source in the USA (up to 70% in the oil) of ricinoleic acid but also of lesquerolic acid, the C20 homologue of ricinoleic acid (14-hydroxy-11-eicosenoic acid). One of the most studied species is
Physaria fendleri, formerly Lesquerella fendleri, Brassicaceae. That plant is a new industrial oilseed crop in the southwestern region of the U.S. can also be used for industry similar to those of ricinoleate and it is amenable to Agrobacterium-mediated transformation to increase the lesquerolic acid production (Wang W et al., Plant Cell Tissue Organ Cult 2008, 92, 165). The identification of triacylglycerol and diacylglycerol species in the seed oil of Physaria fendleri using mass spectrometry has been reported (Lin JT et al., J Am Oil Chem Soc 2013, 90, 1819). In these species, two other hydroxylated fatty acids are found : densipolic acid (12-hydroxy-9,15-octadecadienoic acid) and auricolic acid (14-hydroxy-11,17-eicosadienoic acid) (Smith CR et al., J Org Chem 1962, 27, 3112). Densipolic acid is present in triacylglycerols of Lesquerella lyrata and forms estolide complexes (tetraacylglycerol) when esterified by another normal fatty acid (triacylglycerol-estolides) (Zhang H et al., Ind Crops Prod 2012, 37, 186). Thirteen tetraacylglycerol species have been detected in Physaria fendleri (Lin JT et al., J Am Oil Chem Soc 2013, 90, 1831).
11-Hydroxy hexadecanoic acid, or jalapinolic acid, occurs in jalapin (resin from the rhizoma of Ipomea operculata) and in scammoniae resina (resin from the roots of Convolvulus scammonia). The main components containing jaliponoic acid in these resins are operculinic acids (Ono M et al., Chem Pharm Bull 1990, 38, 2650).
9-Hydroxyacid : another unusual hydroxylated fatty acid which may have many potential oleochemical applications has been discovered in munch seed oil (Dimorphotheca pluvialis) : b-dimorphecolic acid (9-hydroxy,10t,12t-18:2) (Smith CR et al., J Am Chem Soc 1960, 82, 1417). This compound which is also a conjugated diene seems to be a versatile raw material for applications in chemical, pharmaceutical, flavor and fragrance industries since it can be readily dehydrated to a mixture of conjugated triene acids. The 10t12c form of the 9-hydroxy-18:2 (isomeric with dimorphecolic acid) is present in the seed oil of Xeranthemum annuum, Dimorphotheca, Tragopogon, Tagetes, Bidens and Cosmos. This compound was shown to be a partial agonist at PGEl and PGD2 receptors on human platelets (Henry DY et al., Eur J Biochem 1987, 170, 389).
Dimorphecolic acid has been isolated in relatively pure state by supercritical carbon dioxide extraction of munch seed oil (Cuperus FP et al., JAOCS 1996, 73, 1675).
Another isomeric form of dimorphecolic acid, coriolic acid (13-hydroxy-9c,11t-octadecadienoic acid), was reported to be present at high level (70%) in the seed oil of a Coriaraceae Coriaria myrtifolia (Hanseen KS et al., Acta Chem Scand 1967, 21, 301) but also in a Polygonaceae Monnina emerginata (30%) (Phillips BE et al., Biochim Biophys Acta 1970, 210, 353).
Unsaturated oils can contain small amounts of hydroxy derivatives on storage, probably through enzymatic and/or non-enzymatic oxidation.
A wide range of hydroxylated fatty acids is found in sediments but unfortunately these compounds have received little attention from organic geochemists. Long-chain up to C24 and a- and b-monohydroxy acids were observed in a 5000 year-old lacustrine sediment from the English Lake district (Eglinton G et al., Tetrahedron 1968, 24, 5929), and were attributed to microbial oxidation of monocarboxylic fatty acids.
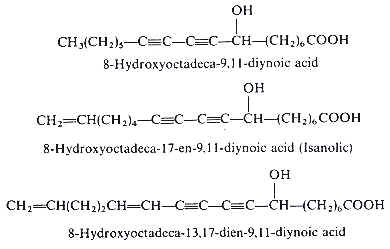
Analytical works on the seed oil of Ongokea gore (Olacaceae) have demonstrated the existence of a variety of hydroxylated diynoic acids (review in Badami RC et al., Prog Lipid Res 1981, 19, 119). Some of them have no other double bond as 8-hydroxyoctadeca-9,11-diynoic acid, others have in addition one double bond as isanolic acid or two double bonds as 8-hydroxyoctadeca-13,17-dien-9,11-diynoic acid.
POLYHYDROXY FATTY ACIDS
A long-chain dihydroxy fatty acid has been found in an Euphorbiaceae Baliospermum axillare seed oil and characterized as 11,13-dihydroxy-tetracos-9t-enoic acid (Husain S et al., Phytochemistry 1980, 19, 75). It was named axillarenic acid.
Two dihydroxy fatty acids have been isolated from floral oils produced by various flowers (Mariza R et al., J Chem Ecol 2007, 33, 1421). These fatty acids (3,7-dihydroxy-eicosanoic and docosanoic acids) were named tetrapedic acids. One of them (3,7-dihydroxy-docosanoic acid) may be di-acetylated, and was named byrsonic acid. These fatty acids were also found acylating a mono-acylated glycerol molecule. These oily compounds are produced by special flower devices (elaiophores) and attract pollinating insects.
Two dihydroxy fatty acids, 15,16-dihydroxy-30:0 and 16,17-dihydroxy-33:0, have also been identified from the acid hydrolysis of the cell residue of Nannochloropsis.
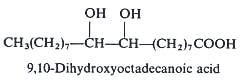
The seed oil of Cardamine impatiens (Cruciferae, Brassicaceae) contains a series of long-chain vicinal dihydroxy fatty acids which make up of about 25% of the oil (Mikolajczak KL et al., JAOCS 1965, 42, 939). This peculiar structure remains unique in phytochemistry. One example with a C18 chain is shown below. Other members of the series have a C20, C22 or a C24 chain but with the hydroxyl groups remaining at the same position with respect to the terminal methyl group. 9,10-dihydroxyoctadecanoic acid was also found at 11% in the seed oil of a Rutaceae Feronia elephantum (Badami RC et al., J Oil Tech Assoc India 1972, 4, 59).
An isomeric form of the previous one but not with vicinal hydroxy groups has been described in the seed oil of a Rutaceae Peganium harmala, 9,14-dihydroxyoctadecanoic acid (Ahmad I et al., Phytochemistry 1977, 16, 1761).
Cutin is known to be a polyester polymer which is insoluble and can be hydrolyzed mainly into a mixture of long-chain C16 and C18 w-hydroxyacids having frequently hydroxyl or epoxy groups in secondary positions. Phellonic acic, 22-hydroxydocosanoic acid, was also detected in cork suberin. Several studies led to tentative models of cutin based on the inter-esterification of w-hydroxyacids, both head-to-tail in a linear form , and cross-linked via the secondary hydroxyls. In some plant species cutin, 16-carbon dihydroxy and 18-carbon trihydroxyacids were detected (Graca J et al., Phytochemistry 2002, 61, 205).
Suberin is a similar type of polyester polymer which contains among other fatty acids a,w-diacids as long-chain monomers esterifying glycerol (Graca J et al., Chem Phys Lipids 2006, 144, 96). Moreover, these diacids (C16, C18, C18:1, and C22) are further esterified by another glycerol molecule or by a hydroxylated fatty acid.
A trihydroxylated oxo-fatty acid, phaseolic acid (2-oxo-5,8,12-trihydroxydodecanoic acid) was shown to stimulate elongation in pea stem segments (Farmer EE, Plant Mol Biol 1994, 26, 1426). This compounds is reminiscent of animal lipoxins.
The 9,10,18-trihydroxyoctadecanoic acid (phloionolic acid) was isolated from the seed oil of Chamaepeuce afra (Mikolajczak KL et al., Lipids 1967, 2, 261) but was also recognized as an important constituent of cork suberin (Holloway PJ, Chem Phys Lipids 1972, 9, 158).
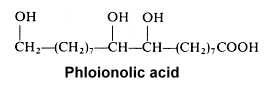
A monounsaturated derivative of phloionolic acid was also detected in some seed oil.
Higher plant cutin and suberin can also be a significant source of esterified C16-C22 a-, b-, and w-monohydroxy and C16 and C18 polyhydroxylated fatty acids in sediments (Cardoso JN et al.,Geochim Cosmochim Acta 1983, 47, 723).
Estolides are dimers formed by a normal fatty acid esterifying a hydroxy fatty acid. They are found mainly in some special triglycerides where they acylate the sn-3 position and formed estolide tetraester triglyceride. They are found in seed oil from Euphorbiaceae. A hydroxy allenic acid (8-hydroxy-5,6-octadienoic acid) was described in an estolide from Sebastiana commersoniana (Spitzer V. et al. Lipids 1997, 32, 549). The w-hydroxyl group was shown to be acylated by a conjugated diene with 10 carbon atoms.
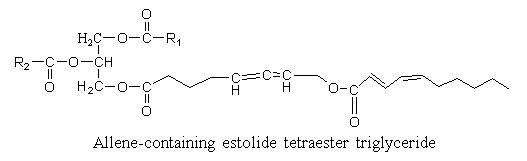
The allenic acid was shown to have antifungal properties (Ohigashi H et al Agr Biol Chem 1972, 36, 1399), the triglyceride being not recommended for animal diet.
Original estolides, named mayolenes, have been isolated from the glandular hairs of a caterpillar (Pieris rapae) (Smedley SR et al., PNAS 2002, 99, 6822).
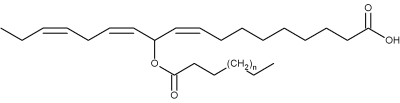
Mayolenes (n = 9 to 15)
These estolides are formed by a 11-hydroxylinolenic acid esterified by a saturated fatty acid (C14-C20). Bioassays demonstrated that they are potent deterrent, playing a defensive role against predators.
Besides classical wax esters, the secretions of the human Meibomius glands (meibum) which are mixed with tears, contain estolides based on a w-hydroxylated fatty acid (mainly from 30 to 34 carbons) acylated on the terminal hydroxyl by oleic acid (18:1n-9) (Butovich IA et al., J Lipid Res 2009, 50, 2471). Due to their amphiphilic anionogenic nature, these compounds may be responsible for stabilization of the tear film lipid layer.
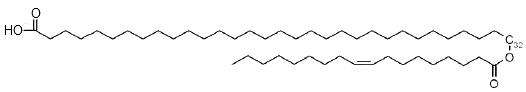
w-Estolide from meibum
Industrially, estolides are now synthesized from vegetal oils and are used as ingredients in various industrial fields. Thus, these new functional fluids have a rapidly growing importance in cosmetics, coatings, and biodegradable lubricants.
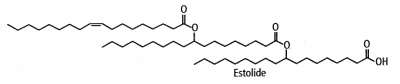
They are largely synthesized from oleic acid warmed with perchloric or sulfuric acid (Cermak SC et al. JAOCS 2001, 78, 557). The average number of fatty acid units added to the first base fatty acid (named "estolide number") varied as a function of reaction temperature. The secondary ester linkages are more resistant to hydrolysis than those of triglycerides, and the unique structure of the estolide results in materials having far superior physical properties than mineral oils and vegetable and petroleum-based oils. They are said to improve intra-fiber moisture retention, to restore elasticity, and prevent mechanical damage. In skin care systems, they provide significant moisturization benefits.
Estolides made from vegetal oils have a good oxidative stability and low-temperature properties. Oxidative stability may be improved in removing the unsaturation of oleic acid and the low-temperature performance may be improved in using oleic acid and various short or middle-chain saturated fatty acids (lauric or myristic acid, coconut oil). Other chemical developments are in progress to obtain molecules with required functional fluid conditions (Cermak S et al., Inform 2004, 15, 515).
The analysis of these compounds may be effected by a combination of gel permeation chromatography, TLC, and gas chromatography (Fehling E, JAOCS 1995, 72, 355).
A kind of estolide has been described in the stem bark of an Euphorbiaceae (Alchorena laxiflora), a plant used in Africa to treat some diseases (Sandjo LP et al., JAOCS 2011, 88, 1153). That compound is formed of a C14 fatty acid with a double bond in the n-3 position esterified with a hydroxylated propionic acid.
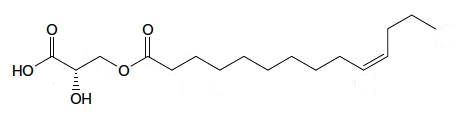
Estolide from Alchorena
Macrolactins are macrolides containing three separate diene structure elements in a 24-membered lactone ring which were first reported to be produced by several marine bacteria (Gustafson K et al., J Am Chem Soc 1989, 111, 7519). Several macrolactin structures have been described in marine bacteria, the simplest one (macrolactin A) is shown below.
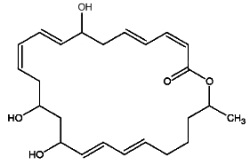
Macrolactin A
These antibiotics may be considered equivalent to a tetrahydroxylated tetracosaenoic acid with an ester bond between the carboxyl group and one of the hydroxyl groups (lactone structure). They are considered to be potent antiviral (against herpes and HIV) and cytotoxic (against melanoma) agents that also have antibacterial activity. It was suggested that the hydroxyl group at C-15 may play an important role in the antibacterial activity of these compounds. There is yet no information about the mechanism of action of this group of compounds.
Parcoblattalactone is a macrocyclic lactone deriving from a 12-carbon fatty acid hydroxylated in position w. This compound is a sex pheromone of the insect Parcoblatta lata, which represents the most abundant arthropod biomass in the pine forests of the southeastern United States (Eliyahu D et al., PNAS, 2012, 109, 490-6). This pheromone will be used for monitoring populations of insects that comprise an important food source for endangered bird species.
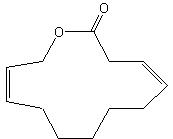
Parcoblattalactone
Lipoxygenase activities give rise to important hydroxylated derivatives mainly from polyunsaturated fatty acids.
Thus, lipoxygenation of 20:4(n-6) results in the formation of a variety of mono- and dihydroxy derivatives. The monohydroxy derivatives consist of the positional isomers 5-hydroxy-20:4, 12-hydroxy-20:4 and 15-hydroxy-20:4. The dihydroxy derivatives include products arising from 5- or 15-lipoxygenation. Double lipoxygenation of 20:4(n-6) at c5 or c12 position give rise to 5,12-diHETE.
In addition to 20:4(n-6), linoleic acid (18:2n-6) is also a substrate for lipoxygenases. In leucocytes (neutrophiles), 13-hydroxy-18:2 (coriolic acid) is produced through enzymatic activity. It appears to be also produced by vascular endothelial cells where it prevents platelet attachment and has vasoconstrictor activity.
It has been shown that 18:3n-3 may be converted by 15-lipoxygenase into mainly 13(S)-OH-18:3 after reduction of the hydroperoxide product. But 18:3n-3 leads also to four di-hydroxylated fatty acid isomers. These compounds are: 9(R),16(S)-dihydroxy-10 E,12 E,14 E-octadecatrienoic acid, 9(S),16(S)-dihydroxy-10 E,12 E,14 E-octadecatrienoic acid, 9(S),16(S)-dihydroxy-10 E,12 Z,14 E-octadecatrienoic acid, and 9(R),16(S)-dihydroxy-10 E,12 Z,14 E -octadecatrienoic acid (Liu M et al., J Lipid Res 2013, 54, 2083). Interestingly, 9,16-dihydroxy-10 E,12 Z,14 E-octadecatrienoic acid isomers exhibit anti-aggregatory properties as other dioxygenated derivatives of PUFA containing an E,Z,E-conjugated triene (PUFA oxygenated trienes or poxytrins).
Allium species (onion and garlic) which are known as folk medicine for the treatment of atherosclerosis and some ulcers, were shown to be rich in two trihydroxylated derivatives of 18:2(n-6) : 9,10,13- and 9,12,13-trihydroxy octadecenoic acids. Furthermore, it was shown that these products have PGE-like activity in in vitro bio-assay tests (Claeys M et al., Prog Lipid Res 1986, 25, 53). Similar products were isolated from roots of Bryone ala, used also for similar medicinal purposes as onion (Panossian AG et al., J Med Plant Res 1983, 47, 17).
Lipoxygenase derivatives of docosahexaenoic acid (DHA), named docosanoids, are known to be formed (mainly 11-OH-DHA) in retinal cells (Bazan N et al., Biochem Biophys Res Comm 1984, 125, 741), but their exact structure and bioactivity were revealed only after 2002. A review of their formation and function in blood and vascular cells may be consulted (Lagarde M, Eur J Lipid Sci Technol 2010, 112, 941). A critical overview on the dihydroxy-docosatrienes may be also consulted (Balas L et al., Biochimie 2014, 99:1-7).
Thus, lipidomic analysis of exudates, vascular, leukocytes and neural cells treated with aspirin have revealed hat DHA was converted into 17R-hydroxy series of dihydroxy- and trihydroxy-docosanoids termed "resolvins" (D-series). They are formed during the resolution phase of acute inflammatory response and are able to counter proinflammation signals (Serhan CN et al., J Exp Med 2002, 196, 1025; Serhan CN et al., Prost Lipid Med 2004, 73, 155).
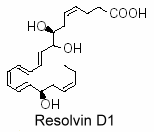
Similarly, EPA is converted into a dihydroxy derivative (resolvin E2) and a trihydroxy derivative (resolvin E1) with five double bonds (E-series) (Oh SF et al., Biochim Biophys Acta 2011, 1811, 737-747).
The metabolism and pharmacological functions of these resolvins have been reviewed (Serhan CN et al., Brit J Pharmacol 2008, 153, S200).
On another hand, brain ischemia was shown to induce a release of DHA from membrane phospholipids which then generates via enzymatic oxygenations novel derivatives named "docosatrienes". These dihydroxy-containing DHA derivatives were termed "neuroprotectins".
The main member of the series was 10,17S-docosatriene (neuroprotectin D1) which was proved to be a potent regulator of inflammation (Marcheselli VL et al., J Biol Chem 2003, 278, 43807).
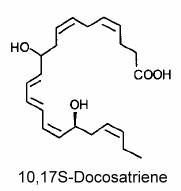
Neuroprotectin D1
Several isomers of protectin D1 were synthesized using soybean lipoxygenase and tested for their ability to inhibit human blood platelet aggregation. It was discovered that the oxygenated products having the E,Z,E-conjugated triene motif and collectively named poxytrins (PUFA oxygenated trienes), might have potent antithrombotic potential (Chen P et al., FASEB J 2011, 25, 382).
The biosynthetic pathway of that DHA derivative in retinal pigment cells and its protective effects from apoptosis induced by an oxidative stress were reported (Mukherjee PK et al., PNAS 2004, 101, 8491). These compounds may be the basis of new therapeutic approaches to enhance photoreceptor survival in retinal degenerations. A review of the rescue and repair processes during photoreceptor cell renewal mediated by neuroprotectin D1 may be consulted (Bazan NG et al., J Lipid Res 2010, 51, 2018).
Some isomers exhibiting the 11t,13c,15t geometry, instead of 11t,13t,15c as in protectin D1 (poxytrins family), are able to inhibit strongly blood platelet aggregation (Chen P et al., FASEB J 2011,25, 382).
Besides 10,17S-docosatriene, the analogue compound 7,17S-docosatriene was shown to be produced during aerobic oxidation of DHA by soybean lipoxygenase (Butovich IA et al., J Lipid Res 2006, 47, 2462). Enzymatic investigations suggest that these compounds might have anti-inflammatory and anticancer activities, which could be exerted, at least in part, through direct inhibition of 5- and 15 lipoxygenase. An overview of their role in brain physiology and a discussion on the potential of using DHA signaling in the development of treatments in patients suffering from stroke have been released by Niemoller TD et al. (Prost Lipid Mediat 2010, 91, 85).
One neuroprotectin and several resolvins have been shown to be biosynthesized by isolated trout brain cells providing the first evidence for the conservation of these structures from fish to humans as chemical signals in diverse biological systems (Hong S et al. Prost Lipid Mediat 2005, 78, 10).
Maresin 1 (7,14-dihydroxydocosa-4 Z ,8,10,12,16 Z ,19 Z -hexaenoic acid) is a new lipoxygenase product from DHA produced in macrophages. It appears as potent as neuroprotectin D1 in its anti-inflammatory activity (Serhan C N et al., J Exp Med 2009, 206, 215).

Maresin 1
Vascular endothelial cells treated with aspirin was shown to convert eicosapentaenoic acid (EPA) into an intermediate product which gives a bioactive compound 5,12,18R-trihydroxy-EPE (resolvin E1).
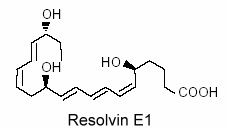
This resolvin was shown to be a potent regulator of PMN cells and inflammation (Serhan CN et al., Prost Lipid Med 2004, 73, 155). New resolvins derived from docosapentaenoic acid of the n-6 family (22:5n-6) have been characterized (Dangi B et al., J Biol Chem 2009, 284, 14744). These products resulting from 15-lipoxygenase activity were determined to be potent anti-inflammatory agents.
A comprehensive review of the metabolism and properties of resolvins, docosatrienes and neuroprotectins may be consulted (Serhan CN et al., Lipids 2004, 39, 1125). An overview of their synthesis and their biological significance have been reviewed (Balas L et al., Prog Lipid Res 2016, 61, 1-18).
Hypoxilins, hydroxy-epoxy derivatives of arachidonic acid are described with lipoxygenase products.
– Thia fatty acids : sulfur-substituted fatty acid analogues are actively synthesized as they are reported to have important pharmacological properties (antiatherosclerosis and antioxidant).
They can have a variable number of carbon atoms and the sulfur atom in different position (3-thia or 4-thia).
The most commonly 3-thia fatty acids studied are presently:
dodeca thia acetic acid CH3-(CH2)11-S-CH2-COOH
tetradeca thia acetic acid CH3-(CH2)13-S-CH2-COOH
These fatty acid derivatives are reported to have triglyceride and cholesterol lowering effects in animal models (Skrede S et al., Biochim Biophys Acta 1997, 1344, 115).
Isomeric epithio stearic acids have been described as minor constituents in canola oil. They were tentatively identified were the 9,12; 8,11; and 7,10 epithio stearic acids (Wijesundera RC et al., JAOCS 1988, 65, 959). The general formula is given below.
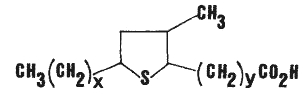
x = 5, 6 or 7 and y = 7, 6 or 5, respectively
– Sulfated fatty acids : New fatty acid derivatives have been isolated from the regurgitant of the grasshopper species Schistocerca americana. These compounds (named caeliferins) are composed of saturated and monounsaturated sulfated a-hydroxy fatty acids in which the w-carbon is substituted with a sulfated hydroxyl group (Alborn HT et al., PNAS 2007, 104, 12976). These compounds have a carbon chain of 1520 carbons and are distributes in varying proportions. Of these, the 16:1 analog is predominant and is also the most active in inducing release of volatile terpenoid compounds when applied to damaged leaves of corn seedlings.
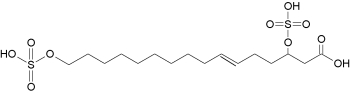
Caeliferin A 16:1
Fatty acid amides are natural products formed by connecting straight-chain, mostly unsaturated, aliphatic acids with various amines by an amide linkage. More than 300 derivatives are known from eight plant families consisting of various combinations of 200 acids with 23 amines.
These compounds are found in nature, but are seldom encountered in fats and oils. As many other nitrogen derivatives of fatty acids (amino acids, hydrazides, acid azides, nitriles, isocyanates, amines), they are of considerable interest and economic importance and have, therefore, been the object of much research and industrial attention mainly in the 50s. They are now produced on a large scale, their chemical features resulting in high surface activities. These compounds are useful as fiber lubricants, detergents, flotation agents, textile softeners, antistatic agents, wax additives, and plasticizers but some of them have also specific biological functions.
Alkamid® is an Internet resource of plant occurring N-alkylamides (http://alkamid.ugent.be/) managed by the Ghent University. Various data of specific molecules can be searched for their origin and their physicochemical properties.
They have a wide variety of biological activities ranging from the characteristic pungent/tingling property and high insecticidal toxicity to significant antifungal, antibacterial, antiprotozoal, molluscicidal, cercaricidal, and acaricidal activity. They also act as plant growth-promoting substances. Some of them possess anti-inflammatory and
analgesic properties and are responsible for immunomodulatory and cannabinomimetic effects (review in: Greger H, Phytochem Rev 2016, 15, 729).
The simple amides may be considered to be products resulting from replacement of the hydroxyl of the carboxyl group with an amino group, RCONH2. The first preparation of stearamide was made in 1882 (Hofmann AW, Ber. 1882, 15, 977) using the procedure of thermal dehydration of ammonium salts discovered by the famous French chemist Dumas (Dumas J, Ann chim phys 1830, 44, 29).
Fatty acid alkanolamides are industrially produced from fatty acids (largely from coconut oil) and alkanolamines, such as ethanolamine, by heating at about 150°C for 6-12 h (Feairheller SH et al., JAOCS 1994, 71, 863).
R-CO-NH-CH2-CH2-OH
They can also be produced from plant erucic acid treated with ammonia.
These compound have a broad spectrum of uses, e.g., in shampoos, detergents, cosmetics, lubricants, foam control agents, and water repellents (Sanders HL, JAOCS 1958, 35, 548), and for the production of nonsticking plastic films and protective coatings.
Simple amides of fatty acids (alkylamides) were shown to be very potent bio-effectors. For example, in chicken chorioallantoid membrane and rat cornea, it was shown that amide of 13-cis-docosenoic acid (erucamide) discovered in the bovine mesentery is an angiogenic factor (Wakamatsu K et al., Biochem. Biophys. Res. Commun., 1990, 168,423). Angiogenic activity (induction of capillary development) was demonstrated by synthetic primary amides of 13-t-docosenoic acid, 18:0, 20:0, 22:0, 20:4n-6, and to a lesser extent of 16:0, 18:1n-9.
The amide of 9-octadecenoic acid (oleamide) was isolated from the cerebrospinal fluid of sleep-deprived cats.
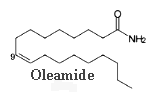
This compound is recognized now to be the endogenous factor inducing sleep in mammals (Cravatt, B F et al., Science, 1995, 268, 1506). Rats treated with oleamide fall asleep. It seems that oleamide may have many other physiological functions, including thermoregulation and sensitivity to pain.
Oleamide is synthesized by the brain cells from oleic acid and ammonium (Sugiura, T et al., Biochem. Mol. Biol. Int., 1996, 40, 93) and its level is regulated by a fatty amide hydrolase which degrades the amide to oleic acid. Apart from its effect on the central nervous system, oleamide modulates the function of the immune cells.
Unexpectedly, oleamide is an important pheromone attractant of the hermaphroditic shrimp Lysmata boggessi.(Zhang D, et al., PLoS One, 2011, 6, e1772064). It is located on the cuticle and its bioactivity is enhanced when incorporated as a pheromone blend with hexadecanamide and methyl linoleate.
Primary amides were identified in the cuticle of Phyllostachys aurea leaves, with a characteristic chain length profile
peaking at C30. The amides were present exclusively in the epicuticular layer and thus at or near the surface, where they may affect plant-herbivore or plant-pathogen interactions (Racovita RC et al., Phytochemistry 2016, 130, 252).
Cerulenin is an antifungal antibiotic discovered in the culture filtrate of Cephalosporium caerulens (Matsumae A et al., J Antibiot (Tokyo) 1963, 16, 236) and shown to inhibit fatty acid biosynthesis (Vance D et al., Biochem Biophys Res Commun 1972, 48, 649). This epoxy-fatty amide has 12 carbon atoms, two double bonds and an epoxy ring. The inhibition of the fatty acid synthase by cerulenin leads to cytotoxicity and apoptosis in human cancer cell lines, effect which suggested a possible cancer treatment. It blocks the synthesis of polyketides in a wide variety of organisms and has a wide range of antimicrobial activity inhibiting the growth of yeast-like fungi, such as Candida, Saccharomyces and Cryptococcus.
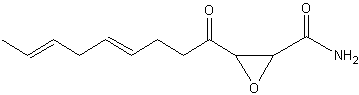
Cerulenin
Many bioactive lipids containing a fatty acid linked to an amine-containing compound are found in animal organisms and are described in the simple lipid section. Much attention has been given to that class of compounds. Briefly, the origins of this research can be traced to 1957 when Kehul FF et al.J Am Chem Soc 1957, 79, 5577) identified N-palmitoylethanolamine as an anti-inflammatory factor present in egg yolk, soybeans, and peanuts. Renewed interest in this and similar N-acylethanolamines arose with the discovery of N-arachidonoylethanolamine (anandamide). An overview of the biochemistry and pharmacology of anandamide has been released (Hansen HS et al., Eur J Lipid Sci Technol 2006, 108, 877).
Thus, simple lipoamino acids with an amide link between one fatty acid and one aminoacid (serine, ornithine, thyrosine, glycine, proline or leucine) or domamine or aminoalcohol (anandamide) are described. <
Other amide-containing lipids (complex lipoamino lipids) are found containing a fatty acid N-linked to an aminoacid (lysine, ornithine, alanine, proline) linked itself to an alcohol (ester link).
A review on the role played by these molecules in pain modulation has been released by Walker JM et al. (Prost Lipid Med 2005, 77, 35).
Fatty acid amides are found in grasses and microalgae. Hexadecanamide and octadecanamide were isolated from the shoots of marine grass Zostera marina (Kawasaki W et al., Phytochemistry 1998, 47, 27). 9-Octadecenamide was identified and quantified (about 2.3% of total fatty acids) among others in the green alga Rhizoclonium hieroglyphicum (Dembitsky VM et al., Phytochemistry 2000, 54, 965). The cyclopropyl fatty amide, grenadamide, was detected from the cyanobacterium Lyngbya majuscula (Sitachitta N et al., J Nat Prod 1998, 61, 681) and a branched-chain fatty amide was isolated from the dinoflagellate Coolia monotis (Tanaka I et al., J Nat Prod 1998, 61, 685).
Several alkylamides have been isolated from Echinacea sp (Asteraceae), one of the most popular medicinal plant used for treatment and prevention of common cold and respiratory tract infections (Woelkart K et al., Planta Med 2007, 73, 615). These alkylamides differ in chain length and unsaturation, many having a diynoic structure. They show structural similarity with anandamide and pharmacological studies have shown that they bind significantly to cannabinoid (CB2) receptors.
A method for the isolation of C18 fatty acid amides from lipid extracts and their analysis by mass spectrometry was reported (Sultana T et al., J Chromatogr A 2006, 1101, 278). The simultaneous quantification of anandamide and other endocannabinoids in brain tissue has been reported (Chen J et al., Chromatographia 2009, 69, 1).
Several benzyl alkylamides (macamides) were isolated from maca (Lepidium meyenii) lipid extract. Tubers of that plant, used as food in Peru and as dietary supplements ("Peruvian ginseng") elsewhere, contain macamides which could have promising biological activities. The simplest structure, described in 2002 (Zhao J et al., Chem Pharm Bull 2002, 50, 988) is shown below.
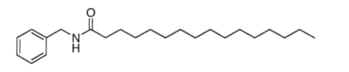
Analogous structures with various chain lengths, unsaturation or substitutions (methoxy or keto group) were also described in the same material (Zhao J et al., J Agric Food Chem 2005, 53, 690).
Similar structures, 11-Cyano or 11-thiocyanato undecanoic acid phenylamide, have been synthesized as corrosion inhibitors to prevent the corrosion of metals in acidic media (Yildirim A et al., Eur J Lipid Sci Technol 2008, 110, 570). These molecules generate a protective layer by adsorption to the metal surface via electrons present on their heteroatoms (0, S, N).
More complex fatty acid amides (one of them is shown below) were discovered in leaves of Chrysanthemum morifolium (Compositae). These isobutylamides, having one or two acetylenic bonds and three or four double bonds, were associated with host-plant resistance against a major insect pest facing greenhouse industry (Frankliniella occidentalis) (Tsao R et al., J Nat Prod 2003, 66, 1229).
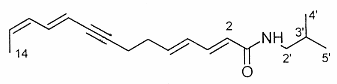
The N-alkylamides dodeca-2,4-dienoic acid (1) and dodeca-2,4,8,10-tetraenoic (2) acid isobutylamides from the plant purple coneflower Echinacea were shown to be likely responsible for the early treatment for colds and as immunostimulants (Gertsch J et al., FEBS Lett 2004, 577, 563).
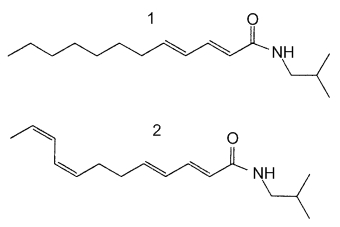
These alkylamides represent a new class of cannabinomimetics which are able to modulate tumor necrosis factor a mRNA expression in human monocytes/macrophages via the cannabinoid type 2 receptor and have immunomodulatory activities (Raduner S et al., J Biol Chem 2006, 281, 14192).
The isobutylamide of deca-trans-2,trans-4-dienoic acid, isolated from the roots of Piper nigrum and various Asteracaea, is a modulator of the sensory neuron function. It is an efficient model compound for sensory studies but also for diabetes, cancer, infection and inflammatory research. The isobytylamide of deca-trans-2,cis-6,trans-8-trienoic acid (Spilanthol), isolated from several species of Spilanthes, is likely responsible for the anesthetic properties of these plants.
Natural alkylamides, called sanshools, are found in the pericarp of the fruit of Szechuan pepper (Menozzi-Smarrito C et al., J Agric Food Chem 2009, 57, 1982). Four sanshools were described in that fruit, they differ from the configuration of one double bond and the length of the polyenic chain (12 or 14 carbons). Two are shown below.
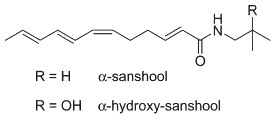
One common characteristic of all these compounds is their agonistic activity on TRPV1, consistent with their burning properties (Sugai E et al., Biosci Biotechnol Biochem 2005, 69, 1951).
A comprehensive review on fatty acid amides may be found on the site of Biochimica (Moscow). Their pharmacological properties were discussed by di Marco V (Biochim Biophys Acta 1998, 1392, 153).
Fatty hydroxamic acids may be regarded as derivatives of both fatty acids and hydroxylamine. Their general formula is R-CO-NHOH.
These compounds are receiving a lot of attention due to their biological activity as inhibitors of cyclooxygenase and 5-lipoxygenase with potent topical antiinflammatory activity (Hamer RR et al., J Med Chem 1996, 39, 246), metal chelators, agent for removing impurities from mineral ores, efficient surfactants in the detergent industry (Masuyama A et al., JAOCS 1987, 64, 764).
Contrary to some short chain hydroxamic acids, fatty hydroxamic acids are not commercially available and are synthesized chemically (Blatt A, Organic synthesis, 1963, 67, vol 2, J Wiley) or by lipase-catalyzed reaction (Suhendra D et al., J Oleo Sci 2005, 54, 33).
Azamacrolides are alkaloids found in defensive droplets from glandular hairs of the pupa of the Mexican bean beetle, Epilachna varivestis (Attygalle AB et al., PNAS 1993, 90, 5204). Chemical studies have shown that the major constituent of this secretion, epilachnene, is the 11-propyl-12-azacyclotetradec-5-en-14-olide. this compound is a vary efficient repellant for ants. Its biosynthesis has been described to be the result of a condensation of oleic acid and serine (Attygalle AB et al., PNAS 1994, 91, 12790).
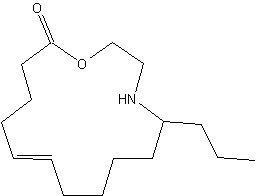
11-Propyl-12-azacyclotetradec-5-en-14-olide
METHOXY and ACETOXY FATTY ACIDS
Fatty acids may be naturally derivatized with a methoxy or an acetoxy group.
Naturally occurring a-methoxy fatty acids were identified in phospholipids from various sponge species living in warm waters : saturated, monounsaturated and diunsaturated 2-methoxylated fatty acids (19 to 24 carbon atoms) were described (Ayanoglu E et al., Lipids 1983, 18, 830; Carballeira NM et al., Lipids 1992, 27, 72; Carballeira NM et al., J Nat Prod 1998, 61, 675; Carballeira NM et al., Lipids 2007, 42, 1047).
Among them, 2-methoxy-5-hexadecenoic acid, 2-methoxy-6-hexadecenoic acid and 2-methoxy hexadecanoic acid were identified in phospholipids of Caribbean sponges.
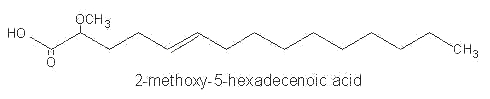
These monounsaturated fatty acids were shown to have antimicrobial activity.
All these compounds were postulated to originate from bacteria in symbiosis with sponges.
Several other saturated and unsaturated 2-methoxylated fatty acids were isolated from phospholipids of a Caribbean sponge (Callyspongia fallax) (Carballeira NM et al., J Nat Prod 2001, 64, 620). The saturated were identified as 2-methoxytetra-, penta- and octadecanoic acids, the monounsaturated as 2-methoxy-6-tetra-, penta- and hexadecenoic acids.
The 2-methoxy-13-methyltetradecanoic acid was identified, together with other 2-methoxylated C15-C16 fatty acids, in the sponge Amphimedon complanata from Puerto Rico (Carballeira NM et al., Lipids 2001, 36, 83). This fatty acid is the methoxylated analog of the bacterial 2-hydroxy-13-methyltetradecanoic acid and was shown to be highly cytotoxic to human cancerous cells (Carballeira NM et al., Chem Phys Lipids 2003, 126, 149). These methoxylated fatty acids could have originated from bacteria in symbiosis with the sponge.
Two 2-methoxy fatty acids with a very long carbon chain (2R,5Z,9Z)-2-methoxy-25-methyl-5,9-hexacosadienoic acid and (2R,5Z,9Z)-2-methoxy-24-methyl-5,9-hexacosadienoic acid were isolated from the Caribbean sponge Asteropus niger and were shown to be effective topoisomerase IB inhibitors (Carballeira NM et al., Lipids 2016, 51, 245–256).
Several saturated 2-methoxylated fatty acids (from C10 to C14) were synthesized and were shown to display some degree of inhibition of Mycobacterium tuberculosis (Carballeira NM et al., Lipids 2004, 39, 675).
Fatty acids methoxylated at other positions have been identified from a few natural sources. The marine cyanobacterium Lyngbya majuscula contain 7-methoxy-4-tetradecenoic acid (Cardellina JH et al., Phytochemistry 1978, 17, 2091), 9-methoxypentadecanoic acid and 15-methoxytricosanoic acid were identified in the red algae Schizymenia dubyi (Barnathan G et al., Phytochemistry 1998, 47, 761). Other acids have originated from bacteria, such as 11-methoxyheptadecanoic and 11-methoxynonadecanoic acids identified in Helicobacter pylori from human gastric mucosae (Inamoto Y et al., J Gastroenterol 1995, 30, 315). 10-methoxyoctadecanoic, 11-methoxyeicosanoic and 13-methoxynonadecanoic acid were detected in the bacterium Thiobacillus (Kerger BD et al., FEMS Microbiol Lett 1986, 38, 67).
Unusual long-chain methoxylated fatty acids were detected in the fungi Blumeria graminis (the causal agent of wheat powdery mildew) (Muchembled J et al., Phytochemistry 2005, 66, 793). They were identified as 3-methoxydocosanoic and 3-methoxytetracosanoic acids.
The phospholipids of the sponge Polymastia gleneni were shown to contain saturated long chain (C-22 to C-30)-acetoxy fatty acids (Ayanoglu E et al., Lipids 1985, 20, 141).
In plants, several 3-acetoxy fatty acids with 14, 16 or 18 carbon atoms are constituents of the partially acetylated acylglycerols found in floral oils of several species of Diascia (Srophulariaceae) (Dumri K et al., Phytochemistry 2008, 69, 1372). In Calceolaria and Lysimachia, a diacylglycerol, (1,2-di-(3-acetoxy-11-octadecenoy)-sn-glycerol) is the major floral lipid.
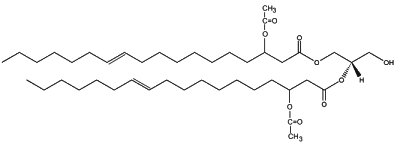
1,2-di-(3-acetoxy-E-11-octadecenoy)-sn-glycerol
From Byrsonima intermedia (Malpighiaceae) floral oil, a di-acetylated fatty acid was isolated, 3,7-diacetoxy-docosanoic acid (bryonic acid) (Reis MG et al., J Chem Ecol 2007, 33, 1421). Several partially acetylated dihydroxy fatty acids could be identified in the floral oil secreted by Malpighia coccigera (Malpighiaceae) (Seipold L et al., Chem Biodiv 2004, 1, 1519). These fatty acids had a chain of 20, 22 or 24 carbon atoms and two hydroxyl groups in various positions, one of them being acetoxylated.
Unexpectedly, these compounds characterize non-volatile oils produced in flowers by special anatomical adaptations (elaiophores) which are collected by pollinating bees, mainly in tropical areas of North and South America (neotropic ecozone). The bees seem to use these lipid secretions (instead of nectar) as provision for their larvae. These structures, generating a "floral syndrome", were described for the first time by Vogel S in 1969 (XI Proc Intl Bot Congress, Seattle, 1969, p. 229. Abstr). In 1987, a review on the ecology of oil flowers and their bees (more than 400 species) listed more than 2400 species of flowering plants (10 families) offering fatty oils (Buchmann SL, Ann Rev Ecol Syst 1987, 18, 343).
A group of unusual triglycerides, in which one of the acyl groups is a vicinal dihydroxy acid with one of the hydroxyl groups acetylated, has been isolated from Cardamine impatiens (Cruciferae) seed oil. Several species were identified : C-18, C-20, C-22, and C-24 hydroxy acetoxy fatty acids (Mikolajczak KL et al., Lipids 1968, 3, 215).
Keto fatty acids are rare but one is well known, 9-keto-2t-decenoic acid, which is an active constituent of the royal jelly (milky substance produced by the worker honey bee).
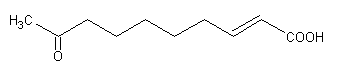
This fatty acid which has a pheromone role, is produced by the mandibular glands of the queen, it attracts and controls the activities of the workers in suppressing the queen-rearing behavior of the worker bees. Several other examples of similar chemicals participate in animal chemoreception (Winston M et al., Am Scientist 1992, 80, 374).
9-keto-2-decenoic acid could be a stimulant of the antibiotic activities against harmful bacteria and fungal infestations.
A new keto fatty acid, 9-keto-13-18:1(26%), was isolated from the seed oil of Smilax macrophylla (Liliaceae) (Daulatabad CD et al., Phytochemistry 1996, 42, 889).
Rosaceae are characterized by some unusual fatty acids, among them the triunsaturated licanic acid, 4-keto-9c11t13t-octadecatrienoic acid, which was described for the first time in oiticica oil extracted in Brazil from Licania rigida (Brown WB et al., Biochem J 1935, 29, 631). This keto derivative of eleostearic acid is present at high concentration (about 60%) in Licania seed oil (Mendelowitz A et al., Analyst 1953, 78, 704). This oil is used as a drying oil for varnishes, and as a component in the manufacture of alkyd resins.
Three long-chain keto fatty acids have been reported at levels from 3 to 13% in the seed oil of a Bignoniaceae Cuspidaria pterocarpa : 15-keto-18-tetracosaenoic, 17-keto-20-hexacosaenoic and 19-keto-22-octacosenoic acids (Smith CR, Lipids 1966, 1, 268). The seed oil of a Papaveraceae Argemone mexicana was shown to contain about 1% of 9-keto- and 11-keto-octacosanoic acids, and a 30 carbon chain keto fatty acid, 11-keto-triacontanoic acid (Gunstone FD et al., Chem Phys Lipids 1977, 20, 331).
The isolation and structure elucidation of an unusual fatty acid with g-oxocrotonate partial structure have been described (Teichert A et al., Z Naturforsch 2005, 60, 25). That compound revealed fungicidal activity against the ascomycete Phytophthora infestans, the causal agent of potato and tomato late blight disease. That ascomycte is known as one of the most destructive pathogens worldwide (Eschen-Lippold L et al., J Agric Food Chem 2009, 57, 9607).
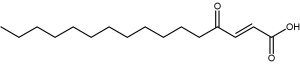
(2E)-4-ketohexadec-2-enoic acid
It has been found that a ketol derivative of linolenic acid, 9-hydroxy-10-keto-12(Z),15(Z)-octadecadienoic acid, is a signal compound in Lemna paucicostata after exposure to stress, such as drought, heat or osmotic stress (Suzuki M et al., Plant Cell Physiol 2003, 44, 35). That compound reacts with catecholamines to generate products that strongly induce flowering (Yokoyama et al., Plant Cell Physiol 2000, 41, 110; Yamaguchi et al., Plant Cell Physiol 2001, 42, 1201).
A new keto acid, 12-keto-5,8,10-heptadecatrienoic acid, is produced in mesenchymal stem cells treated by chemotherapy products and was shown to be related to the induced resistance after treatment. The formation of that keto acid, through the action of cyclooxygenase-1 and thromboxane synthase, could be a target to enhance chemotherapy efficacy in patients (Roodhart JML et al., Cancer Cell 2011, 20, 370).
A 20-carbon keto acid, 15-ketoeicosatetraenoic acid, was discovered to be attached to phosphatidylethanolamine found in monocytes and macrophages (Hammond VJ et al., 2012, 287, 41651). It was shown that that compound is able to activate peroxisome proliferator-activated receptor-g.
OXO FATTY ACIDS
There are a number of aldehydic fatty acids (w-oxo acids) in plants which derive from fatty acid hydroperoxides and play important cell signaling roles.
These molecules form the well known "traumatin" family which includes traumatin (12-oxo-9Z-dodecenoic acid, the precursor of traumatic acid), and autoxidation derivatives (9-hydroxy- and 11-hydroxy-traumatin). Traumatic acid is considered as a plant growth hormone.
t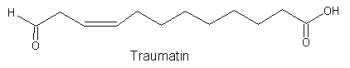
Traumatin hydro-derivatives were shown to be formed by a non-enzymatic oxidation process (Noordermeer MA et al., Biochem Biophys Res Comm 2000, 277, 112). Traumatin and other w-oxo acids were shown to be the products of the successive actions of lipoxygenase and hydroperoxide lyase on linoleic and linolenic acids (Gardner HW, Lipids 1998, 33, 745). The main biological property of traumatin is the ability to stimulate wound healing in plants (Zimmerman et al., Plant Physiol 1979, 63, 536). Traumatin and derivatives are likely to play a role in defense against fungi, bacteria and arthropods (Farmer EE, Plant Mol Biol 1994, 26, 1423).
The homolytic cleavage of fatty acid peroxides by hydroperoxide lyase gives an alcohol (or hydrocarbon) and a w-oxo acid (aldehydic fatty acid) (Gardner HW et al., Plant Physiol 1991, 97, 1059).
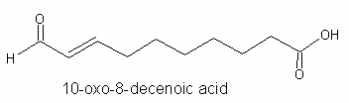
In mushrooms (Psalliota), the production of 10-oxo-8E-decenoic acid from linoleic acid was demonstrated (Wurzenberger M et al., Lipids 1986, 21, 261).
In algae, the cleavage of 13-hydroperoxides of linoleic and linolenic acids produced 13-oxo-9Z-11E-tridecadienoic acid (Vick BA et al., Plant Physiol 1989, 90, 125).
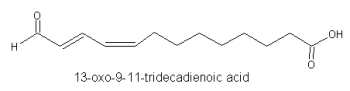
This compound was shown to be also produced by soybean cotyledons (Kondo Y et al., Biochim Biophys Acta 1995, 1255, 9).
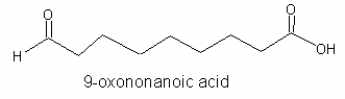
The heterolytic cleavage of 9- and 13-hydroperoxides in higher plants leads to the production of traumatin and 9-oxononanoic acid (Delcarte J et al., Biotechnol Agron Soc Environ 2000, 4, 157).
It was shown that in mammal (rabbit liver) another enzymatic pathway (P-450 and reductase) was also able to generate 13-oxo-9-11-tridecadienoic acid as in algae (Rota C et al., Biochem J 1997, 323, 565).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire