
Aliphatic alcohols occur naturally in free form (component of the cuticular lipids) but more usually in esterified (wax esters) or etherified form (glyceryl ethers). Several alcohols belong to aroma compounds which are found in environmental or food systems (see the website: Flavornet).
They are found with normal, branched (mono- or isoprenoid), saturated or unsaturated of various chain length and sometimes with secondary or even tertiary alcoholic function. An unusual phenolic alcohol is found as a component of glycolipids in Mycobacteria. Some cyclic alcohols have been described in plants.
A classification according to the carbon-chain structure is given below.
The carbon chain may be fully saturated or unsaturated (with double and/or triple bonds), it may also be substituted with chlorine, bromine or sulfate groups. Some acetylenic alcohols have been also described.
Among the most common, some are listed below
|
Formula |
Normal alcohols |
Iso-alcohols |
Anteiso-alcohols |
|
C12H25OH |
1-dodecanol |
10-methyl-1-hendecanol |
9-methyl-1-hendecanol (anteisolauryl alcohol) |
|
C14H29OH |
1-tetradecanol |
12-methyl-1-tridecanol |
11-methyl-1-tridecanol (anteisomyristyl alcohol) |
|
C16H33OH |
1-hexadecanol |
14-methyl-1-pentadecanol (isopalmityl alcohol) |
13-methyl-1-pentadecanol (anteisopalmityl alcohol) |
|
C18H37OH |
1-octadecanol |
16-methyl-1-heptadecanol (isostearyl alcohol) |
15-methyl-1-pentadecanol (anteisostearyl alcohol) |
Free fatty alcohols are not commonly found in epicuticular lipids of insects, although high molecular weight alcohols have been reported in honeybees (Blomquist GJ et al., Insect Biochem 1980, 10, 313). Long-chain alcohols also have been reported in the defensive secretions of scale insects (Byrne DN et al., Physiol Entomol 1988, 13,267). Typically, insects more commonly produce lower molecular weight alcohols. Honeybees produce alcohols of 1722 carbons, which induce arrestment in parasitic varroa mites (Donze G et al., Arch Insect Biochem Physiol 1998, 37, 129). Two female-specific fatty alcohols, docosanol (C22) and eicosanol (C20), which have been found in epicuticle of Triatoma infestans (a vector of Chagas disease in South America), are able to trigger copulation in males (Cocchiararo-Bastias L et al., J Chem Ecol 2011, 37, 246). Hexadecyl acetate is found in the web of some spiders (Pholcidae) to attract females (Schulz S, J Chem Ecol 2013, 39, 1).
Long-chain alcohols (C18, C24, C28) from the femoral glands in the male lizard Acanthodactylus boskianus play a role in chemical communication as a scent marking pheromone (Khannoon ER et al., Chemoecology 2011, 21, 143).
Various fatty alcohols are found in the waxy film that plants have over their leaves and fruits. Among them, octacosanol (C28:0) is the most frequently cited.
Policosanol is a natural mixture of higher primary aliphatic alcohols isolated and purified from sugar cane (Saccharum officinarum, L.) wax, whose main component is octacosanol but contains also hexacosanol (C26:0) and triacontanol or melissyl alcohol (C30:0). Policosanol is also extracted from a diversity of other natural sources such as beeswax, rice bran, and wheat germ (Irmak S et al., Food Chem 2006, 95, 312) but is also present in the fruits, leaves, and surfaces of plants and whole seeds. A complex policosanol mixture has been identified in peanut (Cherif AO et al., J Agric Food Chem 2010, 58, 12143). More than 20 aliphatic alcohols were identified (C14-C30) and four unsaturated alcohols (C20-24). The total policosanol content of the whole peanut samples varied from 11 to 54 mg/100 g of oil.
This mixture was shown to have cholesterol-lowering effects in rabbits (Arruzazabala ML et al., Biol Res 1994, 27, 205). Octacosanol was also able to suppress lipid accumulation in rats fed on a high-fat diet (Kato S et al., Br J Nutr 1995, 73, 433) and to inhibit platelet aggregation (Arruzazabala ML et al., Thromb Res 1993, 69, 321). The effectiveness of policosanol is still questionable but it has been approved as a cholesterol-lowering drug in over 25 countries (Carbajal D et al., Prostaglandins Leukotrienes Essent Fatty Acids 1998, 58, 61), and it is sold as a lipid-lowering supplement in more than 40 countries. More recent studies in mice question about any action on improvement of lipoprotein profiles (Dullens SPJ et al., J Lipid Res 2008, 49, 790). The authors conclude that individual policosanols, as well as natural policosanol mixtures, have no potential for reducing coronary heart disease risk through effects on serum lipoprotein concentrations. Furthermore, sugar cane policosanol at doses of 20 mg daily has shown no lipid lowering effects in subjects with primary hypercholesterolemia (Francini-Pesenti F et al., Phytother Res 2008, 22, 318). It must be noticed that, for the most part, positive results have been obtained by only one research group in Cuba. Outside Cuba, all groups have failed to validate the cholesterol-lowering efficacy of policosanols (Marinangeli C et al., Crit Rev Food Sci Nutr 2010, 50, 259). Independent studies are required before evaluating the exact value of the therapeutic benefits of that mixture.
An unsaturated analogue of octacosanol, octacosa-10, 19-dien-1-ol was synthesized and was as effective as policosanol in inhibiting the upregulation of HMGCoA reductase (Oliaro-Bosso S et al., Lipids 2009, 44, 907). This work opens promising perspectives for the design of new antiangiogenic compounds (Thippeswamy G et al., Eur J Pharmacol 2008, 588, 141). An unsaturated analogue of octacosanol, octacosa-10, 19-dien-1-ol was synthesized and was as effective as policosanol in inhibiting the upregulation of HMGCoA reductase (Oliaro-Bosso S et al., Lipids 2009, 44, 907). This work opens promising perspectives for the design of new antiangiogenic compounds.
1-Octanol and 3-octanol are components of the mushroom flavor (Maga JA, J Agric Food Chem 1981, 29, 1). 3-Octanol is a volatile infochemical present in fungi and recognisable by fungivores (Holighaus G et al., Chemoecology 2014, 24, 57).
Many alcohols in the C10 to C18 range, and their short-chain acid esters are potent sex or aggregation pheromones. They are mainly found as components of specialized defensive glands, pheromone glands or glands of the reproductive system.
A series of C22 up to C28 saturated n-alcohols, with even carbon numbers predominating, and a maximum at C26 and C28, has been identified in the cyanobacterium Anabaena cylindrica (Abreu-Grobois FA et al., Phytochemistry 1977, 16, 351). Several authors have reported high contents of the 22:0 alcohol in sediments where an algal origin is plausible. For example, the major alcohol in a sample of the lacustrine Green River Shale of Eocene age is also 22:0 which comprises over 50% of the alcohols present (Sever JR et al., Science 1969, 164, 1052)
Long-chain alcohols are known as major surface lipid components (waxes) with chains from C20 up to C34 carbon atoms, odd carbon-chain alcohols being found in only low amounts. Very long-chain methyl-branched alcohols (C38 to C44) and their esters with short-chain acids were shown to be present in insects, mainly during metamorphosis. A series of long-chain alkanols (more than 23 carbon atoms) were identified in settling particles and surface sediments from Japanese lakes and were shown to be produced by planktonic bacteria being thus useful molecular markers (Fukushima K et al., Org Geochem 2005, 36, 311).
Cutin and suberin contain as monomer saturated alcohols from C16 to C22 up to 8% of the total polymers. C18:1 alcohol (oleyl alcohol) is also present.
Long-chain di-alcohols (1,3-alkanediols) have been described in the waxes which impregnate the matrix covering all organs of plants (Vermeer CP et al., Phytochemistry 2003, 62, 433). These compounds forming about 11% of the leaf cuticular waxes of Ricinus communis were identified as homologous unbranched alcohols ranging from C22 to C28 with hydroxyl group at the carbon atoms 1 and 3. Very-long-chain compounds were identified and quantified in the petal wax of Cosmos bipinnatus (Asteraceae). The most important were homologous series of alkane 1,2-diols and 1,3-diols, both ranging from C20 to C26 (Buschhaus C et al., Phytochemistry 2013, 91, 249). Relatively little is known about the functions of these compounds in the ecological and physiological fields.
In the leaf cuticular waxes of Myricaria germanica (Tamaricaceae) several alkanediols were identified (Jetter R, Phytochemistry 2000, 55, 169). Hentriacontanediol (C31) with one hydroxyl group in the 12-position and the second one in positions from 2 to 18 is the most abundant diol (9% of the wax). Others were far less abundant : C30-C34 alkanediols with one hydroxyl group on a primary and one on a secondary carbon atom, C25-C43 b-diols and C39-C43 g-diols. Very-long-chain 1,5-alkanediols ranging from C28 to C38, with strong predominance of even carbon numbers, were identified in the cuticular wax of Taxus baccata (Wen M et al., Phytochemistry 2007, 68, 2563). The predominant diol had 32 carbon atoms (29% of the total).
Long-chain saturated C30-C32 diols occur in most marine sediments and in a few instances, such as in Black Sea sediments, they can be the major lipids (de Leeuw JW et al., Geochim Cosmochim Acta 1981, 45, 2281). A microalgal source for these compounds was discovered when Volkman JK et al. (Org Geochem 1992, 18, 131) identified C30-C32 diols in marine eustigmatophytes from the genus Nannochloropsis.
Two nonacosanetriols (7,8,11-nonacosanetriol and 10,12,15-nonacosanetriol) have been isolated from the outer fleshy layer (sarcotesta) of the Ginkgo biloba "fruit" (Zhou G et al., Chem Phys Lipids 2012, 165, 731). They exhibited slight activity of antithrombin and moderate activities of platelet aggregation in vitro.
The chief lipid fraction in the uropygial gland excretion of the domestic hen is a diester wax. The unsaponifiable fraction consists of a series of three homologous compounds, which have been named the uropygiols and identified as 2,3-alkanediols containing 22-24 carbon atoms. These fatty alcohols are esterified by saturated normal C22-C24 fatty acids (Haahti E et al., J Lipid Res 1967, 8, 131).
Some fatty alcohols have one double bond (monounsaturated). Their general formula is:
CH3(CH2)xCH=CH(CH2)y-CH2OH
The unique double bond may be found in different positions: at the C6: i.e. cis-6-octadecen-1-ol (petroselenyl alcohol), C9 i.e cis-9-octadecen-1-ol (oleyl alcohol) and C11 i.e cis-11-octadecen-1-ol (vaccenyl alcohol). Some of these alcohols have insect pheromone activity. As an example, 11-eicosen-1-ol is a major component of the alarm pheromone secreted by the sting apparatus of the worker honeybee. In zooplankton, the cis-11-docosen-1-ol (22:1 (n-11) alcohol) is not only present in high proportion in wax esters (54 to 83%) but may be also predominant in free form (75-94% of free alcohols) in ctenophores (Graeve M et al., Mar Biol 2008, 153, 643). This presence is unexplained because pathways for conversion and catabolism of fatty alcohols in ctenophores are still unknown.
Some short-chain unsaturated alcohols are components of mushroom flavor, such as 1-octen-3-ol, t2-octen-1-ol, and c2-octen-1-ol (Maga JA, J Agric Food Chem 1981, 29, 1). 1-Octen-3-ol is a volatile infochemical present in fungi and recognisable by fungivores (Holighaus G et al., Chemoecology 2014, 24, 57). The liverwort Marchantia polymorpha produces C8 volatiles mainly consisting of (R)-1-octen-3-ol (and octan-3-one) from arachidonic acid upon mechanical wounding (Kihara H et al., Phytochemistry 2014, 107, 42).
An acetoxy derivative of a 16-carbon alcohol with one double bond, gyptol (10-acetoxy cis-7-hexadecen-1-ol), was described to be a strong attractive substance secreted by a female moth (Porthetria dispar, "gypsy moth").
A fatty alcohol with two double bonds, bombykol (tr-10,cis-12-hexadecadien-1-ol), was also shown to be excreted as a very strong attractive substance by the female of silk-worm (Bombyx mori).
![]()
Bombycol
This first discovery of a pheromone was made by Butenandt A et al. (Z Naturforsch 1959, 14, 283) who was formerly Nobel laureate (in 1939) for his work in sex hormones. Another pheromone, 8,10-dodecadienol (codlemone), is secreted by the codling moth Cydia pomonella, has been used for monitoring and mating in apple and pear orchards in the USA and Europe. This molecule was also used to monitor the population of the pea moth Cydia nigricana. Likewise, 7,9-dodecadienol, the female pheromone of the European grapewine moth Lobesia botrana, was used to control this important pest in vineyards.
A fatty triol with one double bond, avocadene (16-heptadecene-1,2,4-triol) is found in avocado fruit (Persea americana) and has been tested for anti-bacterial and anti-inflammatory properties. These properties are likely related with the curative effects of avocado described for a number of ailments (diarrhea, dysentery, abdominal pains and high blood pressure). Several others heptadecanols with one primary and two secondary alcohol functions and with one double or triple bond have been identified in the leaves of Persea americana (Lee TH et al., Food Chem 2012, 132, 921). One or two of these alcohol groups may be acetylated. These compounds may be related to the known antifungal activity of Persea leaves.
Long-chain alkenols (C37 to C39) with 2 to 4 double bonds, the reduced form of the alkenones, have been described in the benthic haptophyte Chrysotila lamellosa (Rontani JF et al., Phytochemistry 2004, 65, 117). al., 1986). C30 to C32 alcohols having one or two double bonds are significant constituents of the lipids of marine eustigmatophytes of the genus Nannochloropsis (Volkman JK et al., Org Geochem 1992, 18, 131). These microalgae could be partially the source of the alkenols found in some marine sediments.
Long-chain a,w-diols, esterified at one or both oxygens with 3-hydroxypropanoic acid, named bruchins, have been described as insect-derived plant regulators which are able to induce the formation of neoplasm on plant (Doss RP et al., PNAS 2000, 97, 6218). One of them is shown below.
![]()
Bruchin A
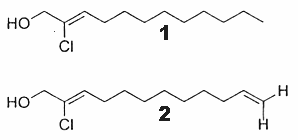
Two chlorinated derivatives of unusual alcohols were described in a red alga Gracilaria verrucosa (Shoeb M et al., J Nat Prod 2003, 66, 1509). Both compounds have a C12 aliphatic chain chlorinated in position 2 and with one double bond at carbon 2 (compound 1 : 2-chlorododec-2-en-1-ol) or two double bonds at carbon 2 and 11 (compound 2 : 2-chlorododec-2,11-dien-1-ol).
Natural acetylenic alcohols and their derivatives have been isolated from a wide variety of plant species, fungi and invertebrates. Pharmacological studies have revealed that many of them display chemical and medicinal properties.
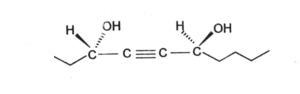
Monoacetylenic alcohols : were isolated from culture of Clitocybe catinus (Basidiomycetes) and the study of their structure revealed the presence of two or three hydroxyl groups (Armone A et al., Phytochemistry 2000, 53, 1087). One of these compounds is shown below.
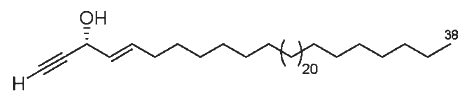
Acetylenic alcohols have been also described in a tropical sponge Reniochaline sp (Lee HS et al., Lipids 2009, 44, 71). One of the two described in that species is shown below, it exhibited a significant growth effect against human tumor cell lines.
Polyacetylenic alcohols : Several examples with different chain lengths, unsaturation degrees, and substitution have been reported from terrestrial plants and marine organisms. Food plants of the Apiaceae (Umbellifereae) plant family such as carrots, celery and parsley, are known to contain several bioactive bisacetylenic alcohols. They are a prominent group of oxylipins and are primarily produced by plants of the families Apiaceae, Araliaceae, and Asteraceae, respectively.
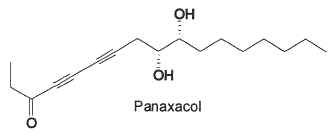
The main plant sources of these compounds are Angelica dahurica, Heracleum sp and Crithmum maritimum (falcarindiol, falcarinol), red ginseng (Panax ginseng) (panaxacol, panaxydol, panaxytriol), Cicuta virosa (virol A), and Clibadium sylvestre (cunaniol). All these compounds display antibiotic or cytotoxic activities.
Polyacetylenes have been isolated from the stems of Oplopanax elatus (Araliaceae), plant used in Korean and Chinese traditional medicine for anti-inflammatory and analgesic purposes (Yang MC et al., J Nat Prod 2010, 73, 801). Among the most efficient in inhibiting the formation of nitric oxide in LPS-induced cells is a seventeen-carbon diyne diol with an epoxy cycle, oploxyne A. Other parent compounds without the epoxy group were also described.
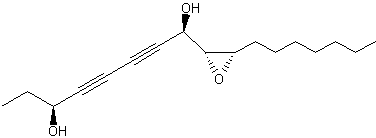
Oploxyne A
A seventeen-carbon diyne fatty alcohol (1,9-heptadecadiene-4,6-diyn-3-ol), was first isolated from Falcaria vulgaris (Bohlmann F et al., Chem Ber 1966, 99, 3552) as well as from Korean ginseng (Takahashi et al., Yakugaku Zasshi 1966, 86, 1053). It was also isolated from carrot (Hansen SL et al. J. Sci. Food Agric. 2003, 83, 1010). Falcarinol has potent anticancer properties on primary mammary epithelial cells and was compared with that of b-carotene. These results might be important in developing new cancer treatments with simple and common vegetables. At high concentrations, falcarinol is capable to induce contact dermatitis.
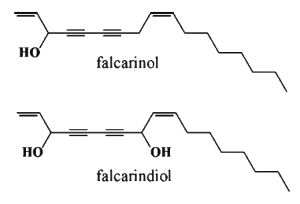
Falcarinol protects the vegetable from fungal diseases, it showed biphasic activity, having stimulatory effects between 0.01 and 0.05 µg per ml and inhibitory effects between 1 and 10 µg per ml, whereas b-carotene showed no effect in the concentration range 0.001100 µg per ml (Hansen SL et al., J Sci Food Agric 2003, 83, 1010). Experiments with macrophage cells have shown that falcarinol (and its C-8 hydroxylated derivative, falcarindiol) reduced nitric oxide production, suggesting that these polyacetylenes are responsible for anti-inflammatory bioactivity (Metzger BT et al., J Agric Food Chem 2008, 56, 3554). Falcarindiol was first reported as phytochemicals in carrots (Daucus carota) (Bentley RK et al., J Chem Soc 1969, 685). Besides falcarinol, falcarindiol, and falcarindiol 3-acetate, nine additional bisacetylene alcohols were identified in Daucus carota (Schmiech L et al., J Agric Food Chem 2009, 57, 11030).
Recent studies on the biological activity of polyacetylenes have indicated their potential to improve human health due to anticancer, antifungal, antibacterial, anti-inflammatory, and serotogenic effects.
Experiments with human intestinal cells demonstrate that aliphatic C17-polyacetylenes (panaxydol, falcarinol, falcarindiol) are potential anticancer principles of carrots and related vegetables (parsley, celery, parsnip, fennel) and that synergistic interaction between bioactive polyacetylenes may be important for their bioactivity (Purup S et al., J Agric Food Chem 2009, 57, 8290). Compounds very similar to falcarinol and extracted from Panax japonicus are potent a-glucosidase inhibitors (Chan HH et al., Phytochemistry 2010, 71, 1360). These inhibitors may potentially reduce the progression of diabetes by decreasing digestion and absorption of carbohydrates.
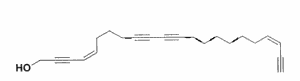
The water dropwort (Oenanthe crocata), which lives near streams in the Northern Hemisphere, contains a violent toxin, cicutoxin, resulting in convultions and respiratory paralysis (Uwai K et al., J Med Chem 2000, 43, 4508).
Cicutoxin
The biochemistry and bioactivity of polyacetylenes are presented in a review (Christensen LP et al., J Pharm Biomed Anal 2006, 41, 683) as well methods for the isolation and quantification of these compounds. A paper has provided a review on falcarinol-type C17-polyacetylenes in carrots and a perspective on their potential as a future contributor to improving human health and well-being (David C. et al., J Agric Food Chem 2015, 63, 9211).
Many other polyacetylenic alcohols were found in primitive marine organisms, such as sponges and ascidians. These invertebrates have no physical defenses and thus they have developed efficient chemical mechanisms such as polyacetylenic metabolites to resist predators and bacteria.
A C36 linear diacetylene alcohol named lembehyne was found in an Indonesian marine sponge (Haliclona sp) (Aoki S et al., Tetrahedron 2000, 56, 9945) and was later able to induce neuronal differentiation in neuroblastoma cell (Aoki S et al., Biochem Biophys Res Comm 2001, 289, 558).

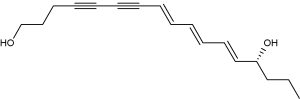
Several polyacetylenic alcohols with 22 carbon atoms were isolated and identified in lipid extract from a Red Sea sponge, Callyspongia sp (Youssef DT et al., J Nat Prod 2003, 66, 679). Their physical study revealed the presence of 4 triple bonds and one, two or three double bonds. The structure of one of these Callyspongenols is given below.
Several di- and tri-acetylenic di-alcohols with a chain of 26 up to 31 carbon atoms, named strongylodiols, have been isolated from a Petrosia Okinawan marine sponge (Watanabe K et al., J Nat Prod 2005, 68, 1001). Some of them have cytotoxic properties.
Several polyacetylenic alcohols with 21 carbon atoms were isolated from a marine ascidian (Polyclinidae) and were determined to have two triple bonds combined with a conjugated dienyne group (Gavagnin M et al., Lipids 2004, 39, 681). Some of them have an additional hydroxyl group or only three double bonds. The structure of one of these molecules is given below.
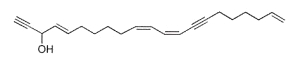
Several brominated polyacetylenic diols with cytotoxic properties were isolated from a Philippines sponge Diplastrella sp (Lerch ML et al., J Nat Prod 2003, 66, 667). One of these molecules is shown below.
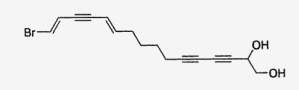
A comprehensive survey of acetylenic alcohols in plant and invertebrates with information on their anticancer activity has been released by Dembitsky VM (Lipids 2006, 41, 883).
Long-chain di-hydroxy alcohols in which both the primary and secondary hydroxyl groups are converted to sulfate esters and one to five chlorine atoms are introduced at various places have been discovered in the alga Ochromonas danica (Chrysophyceae, Chrysophyta) where they constitute 15% of the total lipids (Haines TH, Biochem J 1969, 113, 565). An example of these chlorosulfolipids is given below. There may be several types of chlorine addition : one at R4, two at R3 and R5 or R1 and R2, five at R1 to R5 and six at R1 to R6.
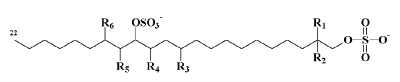
Similar molecules with a 24 carbon chain was also described in Ochromonas malhamensis (review in Dembitsky VM et al., Prog Lipid Res 2002, 41, 315 and in Bedke DK et al., Nat Prod Rep 2011, 28, 15). It was suggested that the chlorosulfolipids replace sulfoquinovosyl diglyceride, since when the later is high the former is low and vice versa. They have been associated with the human toxicity of the mussel-derived lipids (Diarrhetic Shellfish Poisoning).
Several of these chlorosulfolipids have also been identified from more than 30 species of both freshwater and marine algae belonging to green (Chlorophyceae), brown (Phaeophyceae), red (Rhodophyceae) macrophytic algae (Mercer EI et al., Phytochemistry 1979, 18, 457), and other microalgal species (Mercer EI et al., Phytochemistry 1975, 14, 1545).
Some fatty alcohols, such as dodecanol (lauryl or dodecyl alcohol), are used for the manufacture of detergents after sulphonation (by action of SO3 gas). The salt sodium laurylsulfate (or sodium dodecylsulfate) is a detergent and strong anionic surfactant, used in biochemistry and in the composition of cosmetic products (shampoos, toothpastes).
– Mono-methylated alcohols
They are components of the waxes found in several species of Mycobacterium but are not present in other actinomycetes (Minnikin DE et al., Chem Biol 2002, 9, 545). These alcohols are named phthiocerols. Among that family of long-chain secondary alcohols, phthiocerol A, phthiodiolone A and phthiotriol are shown below.
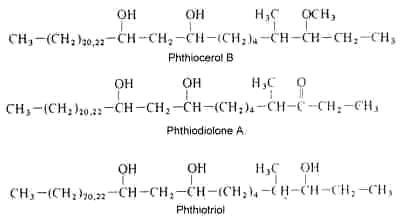
In 1936, Stodola et al. characterized an optically active substance recovered on saponification of purified waxes of Mycobacterium tuberculosis, determined its global formula and proposed to name it phthiocerol (Stodola FH et al., J Biol Chem 1936, 114, 467). In 1959, after several chemical studies, its structure was determined as a mixture of C36 and C34
b-glycols. It has been proposed that the term phthiocerol be reserved for the original 3-methoxy congener (phthiocerol A) and that the term phthioglycol be used to refer to the family of compounds (Onwueme KC et al., Prog Lipid Res 2005, 44, 259).
Some branched alcohols play a role of pheromone in various insects. Among them, 4-methyl-5-nonanol (ferrugineol) is the aggregation pheromone of various species feeding on palm but especially the aggregation pheromone of the red palm weevil, Rhynchophorus ferrugineus (Hallett RH et al., Naturwissenschaften 1993, 80, 328) and 5-methyl-octan-4-ol, the aggregation pheromone of the palmetto weevil, Rhynchophorus cruentatus. These compounds have been proposed for the biological control of these pest insects.
– Polyisoprenoid alcohols
These compounds are fatty alcohols built of several isoprenoid units (C5). They are widespread among eukaryotes and prokaryotes and play important roles in cell function. They have been also found in geological sediments under saturated forms.
The isoprenoid chain may be either saturated or unsaturated.
A general nomenclature of these compounds may be found at the IUPAC web site.
A – Saturated polyisoprenoids (Isopranols)
They have the following general structure :
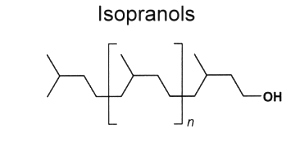
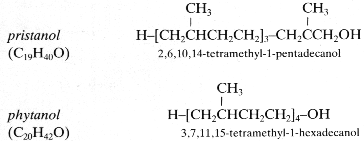
Among the most important saturated isopranols found in plants or in geological sediments are those having two (tetrahydrogeraniol), three (farnesanol), or four (phytanol) isoprenoid units. Pristanol (2,6,10,14-tetramethyl-1-pentadecanol) is tetramethylated but with only three complete isoprenoid units.
B – Unsaturated polyisoprenoids (prenols or polyprenols)
They have the following general structure :
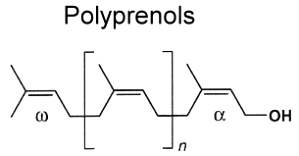
These molecules consist of several up to more than 100 isoprene residues linked head-to-tail, with a hydroxy group at one end (a-residue) and a hydrogen atom at the other (w-end).
Isoprenoid alcohols are also known as terpenols. Search for polyisoprenoid alcohols was initiated with the accidental discovery of solanesol in tobacco leaves (Rowland RL et al., J Am Chem Soc 1956, 78, 4680) and isolation of several polyprenols (C30-C45) in cellulose pulp extracts (Lindgren BO, Acta Chem Scan 1965, 19, 1317). Pioneer studies were summarized in a review by Hemming FW (Biochem Cell Biol 1992, 70, 377).
Head-to-tail assembly of the isoprenyl units produces polymers differing not only in chain length but in geometrical configuration.
Polyprenols are present in several bacteria, where they act as lipid carriers in the biosynthesis of cell surface polymers (Rezanka et al., J Chromatogr A 2001, 936, 95). They have also been described from cyanobacteria. The presence of C35C45 polyprenols has been described in unicellular and filamentous cyanobacteria (Bauersachs T et al., Org Geochem 2010, 41, 867).
Naturally occurring polyprenols can be classified into four groups :
– 1. all trans forms : They have the following structure:
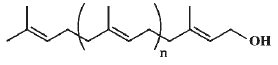
trans-Polyprenol
Some important members of the series are as follows:
| n |
Number of isoprene unit |
Number of carbons |
Name |
| 0 |
2 |
10 |
Geraniol |
| 1 |
3 |
15 |
Farnesol |
| 2 |
4 |
20 |
Geranylgeraniol |
| 3 | 5 | 25 | Geranylfarnesol |
| 7 | 9 | 45 | Solanesol |
| 8 |
10 |
50 |
Spadicol |
Long-chain trans-polyprenol (n>8) have been characterized from Eucommia ulmoides.
Geraniol (from rose oil) is a monoterpene (2 isoprene units). It has a rose-like odor and is commonly used in perfumes and as several fruit flavors. Geraniol is also an effective mosquito repellent. Inversely, it can attract bees as it is produced by the scent glands of honey bees to help them mark nectar-bearing flowers and locate the entrances to their hives.
Farnesol is a sesquiterpene (3 isoprene units). It is the prenol that corresponds to the carbon skeleton of the simplest juvenile hormone described for the first time in insects in 1961 (Schmialek PZ, Z Naturforsch 1961, 16b, 461; Wigglesworth VB, J Insect Physiol 1961, 7, 73). It is present in many essential oils such as citronella, neroli, cyclamen, lemon grass, rose, and others. It is used in perfumery to emphasize the odors of sweet floral perfumes. It is especially used in lilac perfumes. As a pheromone, farnesol is a natural pesticide for mites. The dimorphic fungus Candida albicans has been shown to use farnesol as quorum-sensing molecule (Hornby JM et al., Appl Environ Microbiol 2001, 67, 2982).
Geranylgeraniol is a diterpene (4 isoprene units). Geraniol and geranylgeraniol are important molecules in the synthesis of various terpenes, the acylation of proteins and the synthesis of vitamins (Vitamins E and K). The covalent addition of phosphorylated derivatives of typical isoprenoids, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, to proteins is a process (prenylation) common to G protein subunits. These isoprenylated proteins have key roles in membrane attachment leading to central functionality in cell biology and pathology. It has been demonstrated that a sufficient production of geranylgeraniol is required to maintain endotoxine tolerance in macrophages (Kim J et al., J Lipid Res 2013, 54, 3430).
Solanesol, discovered in tobacco leaves in 1956 (Rowland RL et al., J Am Chem Soc 1956, 78, 4680), may be an important precursor of the tumorigenic polynuclear aromatic hydrocarbons of smoke but is also a possible side chain for plastoquinone. Solanesol is also present in the leaves of other Solanaceae plants including tomato, potato, eggplant and pepper. It has useful medicinal properties and is known to possess anti-bacterial, anti-inflammation, and anti-ulcer activities (Khidyrova NK et al., Chem Nat Compd 2002, 38, 107). Industrially, solanesol is extracted from Solanaceae leaves (about 450 tons in 2008) and used as an intermediate in the synthesis of coenzyme Q10 and vitamin K analogues.
Spadicol was discovered in the spadix (inflorescence) of the Araceae Arum maculatum (Hemming FW et al., Proc R Soc London 1963, 158, 291). Its presence is likely related to its presence in the ubiquinone as the side-chain.
Phytol is a partially saturated diterpene, a monounsaturated derivative of geranylgeraniol which is part of the chlorophyll molecule :
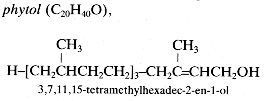
– 2. ditrans-polycis-prenols, such as the bacteria prenol and betulaprenol types. In general, bacteria, as all prokaryotic cells, possess ditrans-polycis-prenols containing between 10 and 12 units, the most abundant being undecaprenol (trivial name bactoprenol).
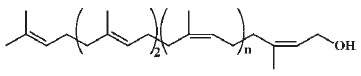
Bacteriaprenol type
Betulaprenols with n = 3-6 were isolated from the woody tissue of Betula verrucosa (Wellburn AR et al., Nature 1966, 212, 1364), and bacterial polyprenol with n = 8 were isolated from Lactobacillus plantarum (Gough DP et al., Biochem J 1970, 118, 167). Betulaprenol-like species with 14 to 22 isoprene units have been discovered in leaves of Ginkgo biloba (Ibata K et al., Biochem J 1983, 213, 305).
Polyisoprenoid alcohols are accumulated in the cells most often as free alcohols and/or esters with carboxylic acids. A fraction of polyisoprenoid phosphates has also been detected, and this form is sometimes predominant in dividing cells and Saccharomyces cerevisiae (Adair WL et al., Arch Biochem Biophys 1987, 259, 589).
– 3. tritrans-polycis-prenols, of the ficaprenol type.
Some of the earliest samples were obtained from Ficus elastica, giving rise to the trivial names ficaprenol-11 and ficaprenol-12 (Stone KJ et al. Biochem J 1967, 102, 325).
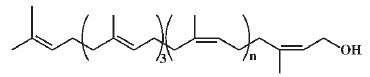
Ficaprenol type
In plants, the diversity of polyprenols is much broader, their chain length covers the broad spectrum of compounds ranging from 6 up to 130 carbon atoms (Rezanka T et al., J Chromatogr A 2001, 936, 95).
– 4. dolichol types, the a terminal is saturated.
Most eukaryotic cells contain one type of polyisoprenoid alcohols with one a-saturated isoprenoid unit (2,3-dihydro polycis-prenols) which have been called dolichol by Pennock JF et al. (Nature 1960, 186, 470), a derivative of prenols. Most of these carry two trans units at the w-end of the chain.
Dolichols (fro the Greek dolikos: long) have the general structure :
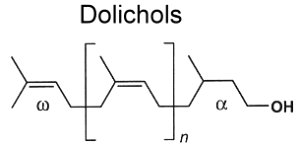
Dolichols isolated from yeast or animal cells consist mainly of seven to eight compounds, those with 16, 18, or 19 isoprenoid units being the most abundant (Ragg SS, Biochem Biophys Res Comm 1998, 243, 1). In human, dolichol-19 (D19, containing 19 isoprene units) is the most abundant species. Dolichol amount was shown to be increased in the brain gray matter of elderly (Pullarkat RK et al., J Biol Chem 1982, 257, 5991). Dolichols with 19, 22 and 23 isoprenoid units were described as early as 1972 in marine invertebrates (Walton MJ et al., Biochem J 1972, 127, 471). Furthermore, the pattern of their distribution may be considered as a chemotaxonomic criterion. It has been reported that a high proportion of dolichols is esterified to fatty acids. As an example, 85-90% of dolichols are esterified in mouse testis (Potter J et al., Biochem Biophys Res Comm 1983, 110, 512). In addition, dolichyl dolichoate has been found in bovine thyroid (Steen L. et al., Biochim Biophys Acta, 1984, 796, 294).
A characteristic shortening of plasma and urinary dolichols in retinitis pigmentosa patients was observed (Wen R et al., J Lipid Res 2013, 54, 3516). Dolichol-18 (D18) became the dominant dolichol species in patients instead of dolichol-19 (D19) in normal individuals.
They are well known for their important role as glycosyl carrier in a phosphorylated form in the synthesis of polysaccharides and glycoproteins in yeast cells, and animals. Dolichyl phosphate is an obligatory intermediate in the biosynthesis of N-glycosidically linked oligosaccharide chains. Conversely, they have been identified as the predominant isoprenoid form in roots (Skorupinska-Tudek K et al., Lipids 2003, 38, 981) and in mushroom tissue (Wojtas M et al., Chem Phys Lipids 2004, 130, 109). Similar compounds (ficaprenols) have the same metabolic function in plants.
The repartition of the various types of polyisoprenoid alcohols between plants and animals and their metabolism have been extensively discussed (Swiezewska E et al., Prog Lipid Res 2005, 44, 235).
Biosynthesis of polyisoprenoid alcohols and their biological role have been reviewed in 2005 (Swiezewska E et al., Prog Lipid Res 2005, 44, 235).
Among the simple phenolic alcohols, monolignols are the source materials for biosynthesis of both lignans and lignin. The starting material for production of monolignols (phenylpropanoid) is the amino acid phenylalanine. There are two main monolignols: coniferyl alcohol and sinapyl alcohol. Para-coumaryl alcohol is similar to conipheryl alcohol but without the methoxy group.
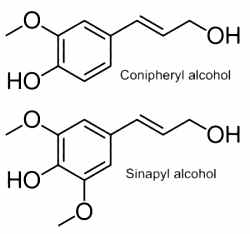
Conipheryl alcohol is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and para-coumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses. Conipheryl esters (conypheryl 8-methylnonanoate) have been described in the fruits of the pepper, Capsicum baccatum (Kobata K et al., Phytochemistry 2008, 69, 1179). These compounds displayed an agonist activity for transient receptor potential vanilloid 1 (capsaicin receptor) as the well known capsaicinoids present in these plant species.
Complex phenolic alcohols (phenolphthiocerol) were shown to be components of Mycobacterium glycolipids which are termed glycosides of phenolphthiocerol dimycocerosate (Smith DW et al., Nature 1960, 186, 887) belonging to the large family of "mycosides". The chain length differs according to the homologues, 18 and 20 carbon atoms in mycosides A, and B, respectively. One of these phenolphthiocerols is shown below.
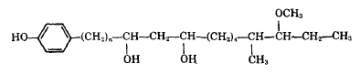
An analogue component but with a ketone group instead of the methoxy group, a phenolphthiodiolone, has been detected in mycoside A (Fournie JJ et al., J Biol Chem 1987, 262, 3174).
An alcohol with a furan group, identified as 3-(4-methylfuran-3-yl)propan-1-ol, has been isolated from a fungal endophyte living in a plant, Setaria viridis (Nakajima H et al., J Agric Food Chem 2010, 58, 2882). That compound was found to have a repellent effect on an insect, Eysarcoris viridis, which is a major pest of rice.
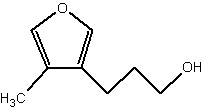
3-(4-methylfuran-3-yl)propan-1-ol
Some cyclic alkyl polyols have been reported in plants. Among the various form present in an Anacardiaceae, Tapirira guianensis, from South America, two displayed anti-protozoal (Plasmodium falciparum) and anti-bacterial (Staphylococcus spp) activities (Roumy V et al., Phytochemistry 2009, 70, 305). The structure shown below is that of a trihydroxy-alcohol containing a cyclohexene ring.
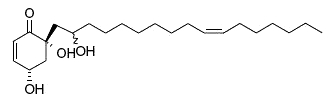
4,6,2′-trihydroxy-6-[10′(Z)-heptadecenyl]-1-cyclohexen-2-one
As emphasized by the authors, external application of the active plant extract or of the purified compounds could represent an accessible therapeutic alternative to classical medicine against leishmaniasis.
Long-chain aldehydes are found in free form, but also in the form of vinyl ether (known as alk-1-enyl ether) integated in glycerides and phospholipids (plasmalogens).
The free aldehydes can be as fatty acids saturated or unsaturated. They have a general formula CH3(CH2)nCHO with n=6 to 20 or greater. The most common is palmitaldehyde (hexadecanal) with a 16 carbon chain. Normal monoenoic aldehydes are analogous to the monoenoic fatty acids.
It must be noticed that an aldehyde function may be found at a terminal (w) position while an acid function is present at the other end of the carbon chain (oxo fatty acids). These compounds have important signaling properties in plants.
Long-chain aldehydes have been described in the waxes which impregnate the matrix covering all organs of plants (Vermeer CP et al., Phytochemistry 2003, 62, 433). These compounds forming about 7% of the leaf cuticular waxes of Ricinus communis were identified as homologous unbranched aldehydes ranging from C22 to C28 with a hydroxyl group at the carbon 3. Long-chain 5-hydroxyaldehydes with chain lengths from C24 to C36, the C28 chain being the most abundant, were identified in the cuticular wax of Taxus baccata needles (Wen M et al., Phytochemistry 2007, 68, 2563). Long-chain aliphatic aldehydes with chain-length from C22 to C30 are also present in virgin olive oils, hexacosanal (C26) being the most abundant aldehyde (Perez-Camino MC et al., Food Chem 2012, 132, 1451).
Aldehydes may be produced during decomposition of fatty acid hydroperoxides following a peroxidation attack. Several aldehydes (hexanal, heptanal..) belong to aroma compounds which are found in environmental or food systems (see the website: Flavornet). Aldehydes (mono- or di-unsaturated) with 5 to 9 carbon atoms are produced by mosses (Bryophyta) after mechanical wounding (Croisier E et al., Phytochemistry 2010, 71, 574). It was shown that they were produced by oxidative fragmentation of polyunsaturated fatty acids (C18, C20). Trans-2-nonenal is an unsaturated aldehyde with an unpleasant odor generated during the peroxidation of polyunsaturated fatty acids. It participates to body odor and is found mainly covalently bound to protein in vivo (Ishino K et al., J Biol Chem 2010, 285, 15302).
It has been demonstrated that sea-lettuce (Ulva species) produces elevated amounts of volatile C10-polyunsaturated aldehydes (2,4,7-decatrienal and 2,4-decadienal) upon tissue damage. These aldehydes are derived from n-3 and n-6 polyunsaturated fatty acids with 20 or 18 carbon atoms including C20:5 n-3 (EPA), C20:4 n-6, C18:4 n-3, and C18:3 n-6. Evidences were presented that lipoxygenase-mediated (11-LOX and 9-LOX) eicosanoid and octadecanoid pathways catalyze the transformation of these fatty acids into short chain hydroxylated fatty acids (Alsufyani T et al., Chem Phys Lipids 2014, 183, 100).
Fatty aldehydes may be determined easily by TLC or gas liquid chromatography (follow that link). The most common method for the determination of aldehydes involves derivatization with an acidic solution of 2,4-dinitrophenylhydrazine to form corresponding hydrazones followed by HPLC separation and UVVIS detection. An optimized derivatization procedure for the determination of aliphatic C1-C10 aldehydes has been described (Stafiej A et al., J Biochem Biophys Meth 2006, 69, 15).
Other short-chain aldehydes (octadienal, octatrienal, heptadienal) are produced via a lipoxygenase-mediated pathway from polyunsaturated fatty acids (mainly C16 and C20) esterifying glycolipids in marine diatoms (D’Ippolito G et al., Biochim Biophys Acta 2004, 1686, 100). In nature, processes producing the disruption of phytoplankton cells are viral infection, grazing or/and cell lysis during senescence. Heighteen species of diatoms have been shown to release unsaturated aldehydes (C7:2, C8:2, C8:3, C10:2, and C10:3) upon cell disruption (Wichard T et al., J Chem Ecol 2005, 31, 949). The analyzis of the spatial distribution of the aldehydes produced by the phytoplankton in the Atlantic Ocean surface has shown that the total potential fatty adehyde concentrations ranged from zero to 4.18 pmol from cells in 1 L with, besides octadienal and decadienal, heptadienal being the most common (Bartual A et al., Mar Drugs 2014, 12, 682).
Several short-chain aldehydes were shown to induce deleterious effects on zooplankton crustaceans and thus limiting the water secondary production (birth-control aldehydes) (D’Ippolito G et al., Tetrahedron Lett 2002, 43, 6133). In laboratory experiments, three decatrienal isomers produced by various diatoms were shown to arrest embryonic development in copepod and sea urchins and have antiproliferative and apoptotic effects on carcinoma cells (Miralto A et al., Nature 1999, 402, 173). Later, the copepod recruitment in blooms of planktonic diatom was shown to be suppressed by ingestion of dinoflagellate aldehydes (Nature 2004, 429, 403). It was demonstrated that diatoms can accurately sense a potent 2E,4E/Z-decadienal and employ it as a signaling molecule to control diatom population sizes (Vardi A et al., PLoS Biol 2006, 4, e60). This aldehyde triggered a dose-dependent calcium transient that has derived from intracellular store. Subsequently, calcium increase led to nitric oxide (NO) generation by a calcium-dependent NO synthase-like activity, resulting in cell death in diatoms.
Myeloperoxidase-derived chlorinated aldehydes with plasmalogens has been reported. Thus, the vinyl-ether bond of plasmalogens is susceptible to attack by HOCl to yield a lysophospholipid and an
a-chloro-fatty aldehyde (Albert CJ et al., J Biol Chem 2001, 276, 23733). For example, 2-chloro-hexadecanal is formed by HOCl attack on the plasmalogen 1-O-hexadec-1′- enyl-sn-glycero-3-phosphocholine. Similarly, 2-chloro-octadecanal is formed from 1-O-octadec-1′-enyl-sn-glycero-3-phosphocholine.

2-Chloro-hexadecanal
Both these chloro-fatty aldehydes have been detected in neutrophils activated with PMA (Thukkani AK et al., J Biol Chem 2002, 277, 3842) and in human atherosclerotic lesions (Thukkani AK et al., Circulation 2003, 108, 3128). Furthermore, 2-chlorohexadecanal was shown to induce COX-2 expression in human coronary artery endothelial cells (Messner MC et al., Lipids 2008, 43, 581). These data suggest that 2-chlorohexadecanal and possibly its metabolite 2-chlorohexadecanoic acid, both produced during leukocyte activation, may alter vascular endothelial cell function by upregulation of COX-2 expression.
Long after the demonstration of the presence of iodinated lipids in thyroid (besides iodinated aminoacids), it was shown that the major iodinated lipid formed in thyroid when incubated in vitro with iodide was 2-iodohexadecanal (Pereira A et al, J Biol Chem 1990, 265, 17018). In rat and dog thyroid, 2-iodooctadecanal was determined to be more abundant that the 16-carbon aldehyde. These compounds, which are thought to play a role in the regulation of thyroid function, were recently shown to be formed by the attack of reactive iodine on the vinyl ether group of PE plasmalogen. This attack generates an unstable iodinated derivative which breaks into lysophosphatidylethanolamine and 2-iodo aldehydes (Panneels V et al, J Biol Chem 1996, 271, 23006).
In some bacteria, aldehyde analogs of cyclopropane fatty acids were described.
Several fatty aldehydes are known to have pheromone functions. Studies in African and Asian countries have shown that the use of 10,12-hexadecadienal could be effective for control of the spiny bollworm Earias insulana, a cotton pest. The sex pheromone of the navel orange worm, Amyelois transitella, 11,13-hexadecadienal, is usually used in the control of this citric pest.
A branched saturated aldehyde (3,5,9-trimethyldodecenal, stylopsal) has been identified as a female-produced sex pheromone in Stylops (Strepsiptera), an entomophagous endoparasitic insect (Cvacka J et al., J Chem Ecol 2012, 38, 1483).
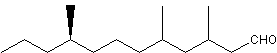
Stylopsal
Several isoprenoid aldehydes are important in insect biology as pheromones and in botany as volatile odorous substances. Some examples are given below:
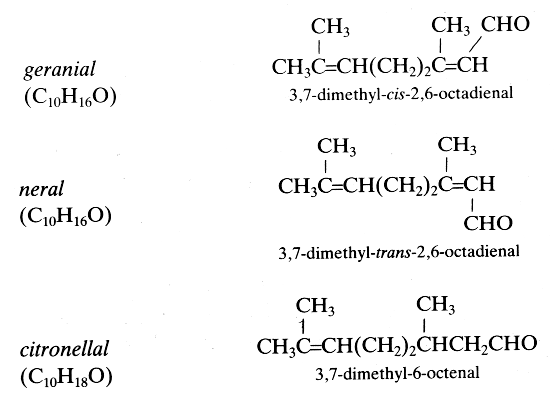
These three terpenic aldehydes are produced in large amounts by the mandibular glands of ants and may function as defensive repellents (Regnier FE et al., J Insect Physiol 1968, 14, 955). In contrast, the same molecules have a role of recruiting pheromones in honeybees
Citral, a mixture of the tautomers geranial (trans-citral) and neral (cis-citral) is a major component (more than 60%) of the lemongrass (Cymbopogon flexuosus) oil. Lemongrass is widely used, particularly in Southeast Asia and Brazil, as a food flavoring, as a perfume, and for its medicinal properties (analgesic and anti-inflammatory). It was found that citral is a major suppressor of COX-2 expression and an activator of PPAR a and g (Katsukawa M et al., Biochim Biophys Acta 2010, 1801, 1214)
It was demonstrated that damaged leaves released 2-hexenal, among other C6-volatile aldehydes, produced from the catalytic activity of hydroperoxide lyase (Turlings TC et al., Proc Ntl Acad Sci USA 1995, 92, 4169). These compounds, considered as signal molecules, can trigger several responses in neighboring plants and may also act as antimicrobial agents (Farmer EE, Nature 2001, 411, 854).
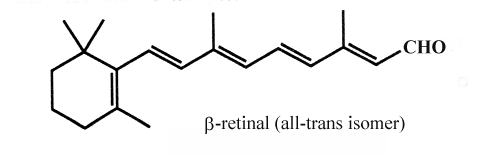
One important constituent of this group of aldehydes is retinal, one active form of vitamin A involved in the light reception of animal eyes but also in bacteria as a component of the proton pump.
 .
.
Retinal exist in two forms, a cis and a trans isomer. On illumination with white light, the visual pigment, rhodopsin is converted to a mixture of a protein (opsin) and trans-retinal. This isomer must be transformed into the cis form by retinal isomerase before it combines again with opsin (dark phase). Both isomers can be reduced to retinol (vitamin A) by a NADH dependent alcohol dehydrogenase. Retinol is stocked in retina mainly in an acylated form.
Cinnamic aldehyde (cinnamaldehyde) is the key flavor compound in cinnamon essential oil extracted from Cinnamomum zeylanicum and Cinnamomum cassia bark. Investigations have revealed than that benzyl aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells (Wondrak GT et al., Molecules 2010, 15, 3338). Cinnamic aldehyde may therefore represent a precious chemopreventive dietary factor targeting colorectal carcinogenesis.
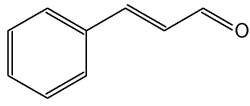
Cinnamic aldehyde
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire