
![]()
(PHYLLOQUINONE)
The existence of the vitamin K group was discovered by H. Dam (Biochim. Zeitschr. 1929, 215, 475) in studying cholesterol metabolism in chicks. He noted a new deficiency syndrome in the young birds fed a fat deficient diet. The characteristic features were a lengthened blood clotting time, anemia and hemorrhage. Ten years later, E.A. Doisy‘s group (Binkley et al., J. Biol. Chem. 1939, 130, 219) succeeded in isolating the vitamin from hexane extracts. Although at the time the structure had not been established, chemical and physical properties were correctly attributed to a substituted 1,4-naphthoquinone. Subsequent works by H. Dam, rewarded with the Nobel prize for medicine in 1943 "for his discovery of vitamin K", with E.A. Doisy "for his discovery of the chemical nature of vitamin K", led to the characterization of the molecule he termed vitamin K (Koagulationsvitamin).
Vitamin K1 was named phylloquinone since it is an indirect product of photosynthesis in plant leaves where it occurs in chloroplasts and participates in the overall photosynthetic process. The methyl naphthoquinone ring (chromene) has a phytyl side chain (partially saturated poly-isoprenoid alcohol).
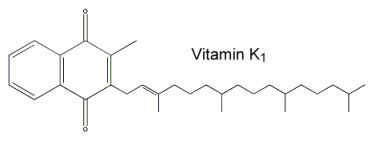
Vitamin K (its reduced form) is necessary for post-translational modification of coagulation factors II (prothrombin), VII, IX and X in the liver. In the presence of the reduced form of vitamin K (dihydro vit K or vitamin K hydroquinone) formed by the action of a reductase enzyme on phylloquinone, certain glutamic acid residues of the nascent polypeptides are converted to g-carboxyglutamic acid by a carboxylase enzyme. This confers the ability to bind calcium, a property essential for the physiological function of these proteins. Vitamin K is also involved in the activation of a protein, the matrix Gla protein (MGP), an inhibitory factor which is highly expressed by vascular smooth muscle cells and regulates vascular calcification (Proufoot D et al., Nephrology 2006, 11, 455). The role of vitamin K and the potential antagonism by anticoagulants have been reviewed on the basis of bone health and osteroporosis (Pearson DA, Nutr Clin Pract 2007, 22, 517).
During the reaction, a molecule of vitamin K epoxide (vit K 2,3-oxide) is formed which is further reduced, mainly by cellular thiol reagents, back to vitamin K1 (phylloquinone).
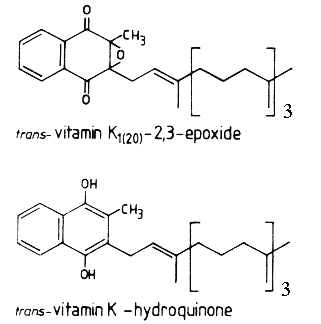
Vitamin K1 (MW: 450.7) is a yellow viscous oil with a maximum absorbance at 248 nm (molar abs coeff.:19900, the same for menaquinones), soluble in ethanol, hexane, chloroform and vegetable oils. Vit K1 is sensitive to sunlight (destroyed after one hour), unaffected by diluted acids but destroyed by basic solution and transformed by reducing agents. Vitamin K1 oxide (MW: 466.7) is much more stable to light and soluble in the same solvents as Vitamin K1. It has a maximum absorbance at 259 nm (molar abs. coeff.: 6170).
The K vitamins are subject to side-chain structural isomerization, and naturally occurring K1 is found exclusively as the biologically active 2′-trans-isomer. However, biological samples may contain some quantities of the inactive cis-isomer. Thus, its amount must be estimated in establishing reliable food databases.
| Food | Concentration (mg/100 g) |
| kale | 800 |
| spinach | 380 |
| brocoli | 240 |
| Brussel sprout | 180 |
| cabbage | 145 |
| asparagus | 100 |
| peas | 40 |
| avocado | 40 |
| lettuce | 35 |
| kiwi | 25 |
| soybean oil | 193 |
| rapeseed oil | 127 |
| cottonseed oil | 60 |
| olive oil | 55 |
Phylloquinone is poorly represented in fruits (1 to 30 ng/g) except avocado (400 ng/g) and kiwi (250 ng/g). Grain products have also very low levels of vitamin K1 (1 to 70 ng/g) . Animal products including eggs do not appear to contain appreciable amounts of vitamin K1 (less than 50 ng/g) and less than 10 ng/g are found in fish and shellfish. High amounts are found in butter (up to 1 µg/g) but lower amounts in cheese (20-100 ng/g). In contrast, a high diversity of menaquinones are present in dairy products.
In animal tissues, phylloquinone is distributed in liver but also in heart, but is present at low concentrations in brain (Thijssen H et al., J Nutr 1996, 126, 537).
An adequate intake for a 25-year old male for Vitamin K is about 120 micrograms/day.
VITAMIN K2
(MENAQUINONES)
A second vitamin K (vit K2) was isolated very early from putrefied fish meal as a product of microbial synthesis (McRee RW et al., J. Am. Chem. Soc. 1939, 61, 1295). It has the same absorption peaks in UV region as vitamin K1 but with a slightly lower intensity (molar abs. coeff. at 248 nm: 11800). Vitamin K2 has a poly-isoprenoid unsaturated side-chain of various length, with isoprene units varying from 4 to 13. These compounds are called menaquinones-n or MK-n.
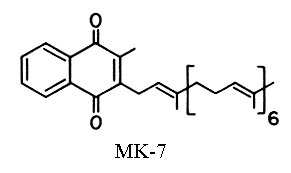
As an example of the various menaquinone forms, MK-7 has 6+1=7 isoprenoid units or 35 carbons in the side chain and can be called vitamin K2(35) or menaquinone-7. This could be called also 2-methyl-3-all-trans-farnesyl digeranyl-1,4-naphthoquinone. Farnesol and geraniol are the alcohols with 3 (15 carbons) and 2 (10 carbons) isoprenoid units, respectively. One of the first menaquinones discovered was MK-6 (Isler, 1958) which can be called 2-methyl-difarnesyl-1,4-naphthoquinone. Most menaquinone-containing organisms contain a series of menaquinones, the major homologues with n=6 to 9 constituting about 90% of the total.
Among human foods, dairy products are particularly rich in menaquinone species. As an example, a common cheese (Camembert) contains about 40 ng/g of vitamin K1 but about 600 ng/g of menaquinones (less than 2% MK5, MK6 and MK7, 9% MK4, 27% MK8 and 62% MK9).
A large survey of menaquinones has been done in various fermented dairy products (Manoury E et al., J Dairy Sci 2013, 96, 1335). It was observed a wide diversity of vitamin K2 contents from undetectable to 1,100 ng/g of product, and a remarkable diversity of menaquinone forms among products. The major form was menaquinone (MK)-9, and contents of MK-9 and MK-8 forms were correlated, that of MK-9 being around 4 times that of MK-8, suggesting that microorganisms able to produce MK-9 also produce MK-8. This was not the case for the other menaquinones, which were produced independently of each other. Finally, no obvious link was established between MK-9 content and fat content or pH of the fermented dairy products.
Natto (a traditional Japanese soybean-based food) is rich in menaquinone-7 (1 mg/100 g) (Schurgers LJ et al., Haemostasis 2000, 30, 298), higher than in cheese (between 40 and 50 mg/100 g).
In animal tissues, menaquinones (mainly MK-4) are present at concentrations exceeding those of phylloquinone in pancreas, salivary gland and brain (Thijssen H et al., J Nutr 1996, 126, 537). MK-4 accumulation in non-hepatic organs was shown to result from a synthesis rather than an uptake from the gut. MK-4 concentration (about 80 times that of phylloquinone) was shown to be correlated with sphingolipid concentrations in rat brain (Carrie I et al., J Nutr 2004, 134, 167). That association suggests a role of that menaquinone in the brain function since the vitamin K status has been shown to influence the brain sulfatide metabolism in young mice and rats (Sundaram KS et al., J Nutr 1996, 126, 2746).
It was shown by oral administration to vitamin K-deficient chicks that isoprenologs with 3 to 5 isoprenoid groups in either menaquinone or phylloquinone type compounds have maximum biological activity.
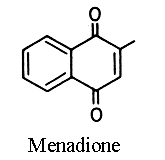
2-methyl-1,4-naphthoquinone or menadione is called also vitamin K3. It has the same physiological activity in
vivo as phylloquinone by alkylation in position 2 with an isoprenoid chain in the liver. As it is a lipid soluble molecule, its activity depends on the presence of lipids in the diet to promote absorption. Menadione sodium bisulfite complex is water soluble and thus is used as food additive in vitamin mix for animals.
Many members of the Boraginaceae family produce naphthoquinone lipids in their roots. They are colored substances derived from phenylpropanoid and isoprenoid precursors. Plants of the borage family (Boraginaceae) are distributed worldwide and the naphthoquinones from many of these plants have been used in diverse cultures as colorants for cosmetics, foods, and for medicinal applications, including antitumor, antiinflammatory, and antimicrobial agents. They are all derivatives of shikonin and its enantiomer alkannin from the European dye plant Alkanna tinctoria.
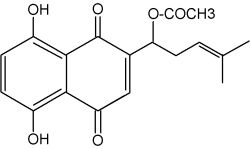
Acetyl shikonin
Because shikonin and its derivatives have biological activity against microorganisms, they compounds may play a role in plant defense in the rhizosphere. More polar naphthoquinones have been shown to function in allelopathy (juglone; Binder et al., Phytochemistry 1989, 28, 2799) and plant-insect interactions. Several biotic factors (fungus, bacteria) increases the amount of total pigment, changes the ratios of derivatives produced, and initiates production of pigment de novo in epidermal cells (Brigham LA et al., Plant Physiol 1999, 119, 417). Shikonin derivatives function as both preformed and inducible microbial inhibitors regulated in a cell-specific manner to maximize the effect of highly toxic substances with minimal expense and harm to the plant.
Various analogues of shikonin have been isolated from a Boraginaceae, Alkanna cappadocica (Sevimli-Gur C et al., J Nat Prod 2010, 73, 860). They are all cytotoxic when tested on cancer cell lines.
Dehydro-alpha-lapachone is one of the numerous naphthoquinones isolated from several trees of the bignoniaceae family (Schmeda-Hirschmann et al., Z Naturforsch 2003, 58c, 495). The main source is the wood of Tabebuia heptaphylla, named "lapacho" in Paraguay. That wood is a traditional medicine used to treat wounds, inflammation and cancer (anti-vascularisation).
Dehydro-alpha-lapachone
Biological activities reported for lapachone comprises molluscicidal and trypanocidal effect. Lapachone can be considered a potential lead for the development of drugs to treat multidrug-resistant cell lines with lower expression of topoisomerase II (Krishnan P et al., Cancer Chemoth Pharm 2001, 47, 187). It was also shown to have antivascular activity in inhibiting vessel regeneration, interfering with vessel anastomosis (Schmeda-Hirschmann G et al., Z Naturforsch 2003, 58c, 495).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire