
FATTY ACIDS
Carboxylic acids occur in many molecular forms. At first It must be recalled that if the majority of the fatty acids found in lipids are monocarboxylic acids, some of them are dicarboxylic and constitute important metabolic or oxidation products of the previous ones.
Several hundreds of forms have been identified but the number occurring frequently in the common lipids is much fewer (from 10 in plants to about 20 in animal tissues). Several fatty acids, free or esterified (methyl butyrate, ethyl octanoate, dodecanoic acid…), belong to aroma compounds which are found in environmental or food systems (see the website: Flavornet). The analyzed fatty acid profiling of plants or microalgae have been used to classify them into distinct taxonomic orders with respect to their phylogenetic classification (Sahu A et al., Phytochemistry 2013, 89, 53). That experimental approach has been reported as a tool for studying the chemotaxonomic features in various species macro- and microalgae.
Fatty acid methyl and ethyl esters are known to be present in the plasma of patient with liver dysfunction following ethanol ingestion (Aleryani SL et al., Clin Chim Acta 2005, 359, 141; Politi L et al., Anal Biochem 2007, 368, 1).
Triglycerides from various vegetable oils give through transesterification a mix of fatty acid esters which is now used increasingly as a substitute of diesel fuel and is named Biodiesel.
The world production of fatty acids from the hydrolysis of natural fats and oils totaled about 4 million metric tons per year. Fatty acids are ultimately consumed in a wide variety of end-use industries (rubber, plastics, detergents…). As it is a good indication of the overall economic performance of a region, the consumption of fatty acids has tended to approximate the growth in the GNP of the region of their consumption. Fatty acids make up the greatest proportion of the current consumption of raw material in the chemical industry. The extent of the chemical reactions which are used to transform these renewable materials has been summarized (Bierman U et al., Angew Chem Int Ed 2000, 39, 2206). A short survey of oil crop platforms to be considered for either multi-purpose or technical oils production has been reviewed in 2009 (Carlsson AS, Biochimie 2009, 91, 665).
To describe precisely the structure of a fatty acid molecule, one must give the length of the carbon chain (number of carbon), the number of double bonds and also the exact position of these double bonds, this will define the biological reactivity of the fatty acid molecule and even of the lipid containing the fatty acids studied.
Most fatty acids are straight-chain compounds with the most frequently an even number of carbon atoms. Odd-numbered fatty acids are mostly frequent in bacteria and lower plants or animals (review in : Rezanka T et al., Prog Lipid Res 2009, 48, 206). Chain-lengths range is from 2 to 80 but commonly from 12 up to 24. With a chain length from 2 to 6 (or 4) they are called short-chain, from 8 (or 6) to 10 they are called medium-chain and 12 up to 24 they are called long-chain fatty acids. Their physical and biological properties are related to this partition in 3 classes.
An extensive review on the biochemical mechanisms of fatty acid elongation may be consulted for further information (Leonard AE et al., Prog Lipid Res 2004, 43, 36).
Fatty acids are simple in structure and even with their derivatives can be subdivided into well-defined families:
Among straight-chain fatty acids, the simplest are referred to as saturated fatty acids. They have no unsaturated linkages and cannot be altered by hydrogenation or halogenation. When double bonds are present, fatty acids are said unsaturated, monounsaturated (MUFA) if only one double bond is present and polyenoic (or polyunsaturated fatty acids = PUFA) if they have two or more double bonds generally separated by a single methylene group (methylene-interrupted unsaturation). In recent physiological works, the last class is used only for fatty acids with three up to six double bonds as those found in fish oil or in brain tissue. Some uncommon polyunsaturated fatty acids have two adjacent double bonds separated by more than one methylene group, they are named polymethylene-interrupted fatty acids.
In some animals, but mainly in plants and bacteria, fatty acids may be more complex since they can have an odd number of carbon atoms, branched chains or contain a variety of other functional groups, including acetylenic bonds, epoxy-, hydroxy– or keto groups and even ring structures (cyclopropane, cyclopropene, cyclopentene, furan, and cyclohexyl) or a coenzyme A moiety (acyl CoA).
Except fatty acyl-CoA, we have based our classification of fatty acids first on the type of carbon chain : either straight (or normal), or branched, or containing a carbon ring. In each category, subdivisions are created according to the functional groups substituted on the carbon chain.
To describe the unsaturated fatty acids, two ways are offered:
The chemist’s terminology:
The carbon atoms are counted from the carboxyl group which put the emphasis on the double bond closest to this group.
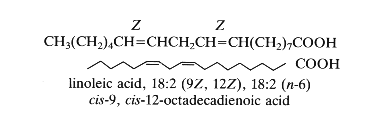
As an example: 18:2 D9,12 or cis-9, cis-12-octadecadienoic acid, the trivial name: linoleic acid. The double bonds have usually a Z (cis) configuration but can have also a E (trans) configuration.
The biochemist’s and physiologist’s terminology:
Holman RT proposed in 1964 a new numbering system for the unsaturation of fatty acids, the "omega nomenclature". The double bonds are counted from the methyl group determining the metabolic family, noted by wx (w for the terminal carbon) or better n-x (n for the total number of carbon, x being the position of the distal double bond) . The other double bonds are deduced from the first one by adding 3 (this is the most frequent structure, non-conjugated fatty acids, but sometimes by adding 2, these double bonds are said conjugated).
Thus, linoleic acid or cis-9, cis-12-octadecadienoic acid is also named in the shorthand nomenclature 18:2 (n-6). This compound has 18 carbon atoms, 2 double bonds and 6 carbon atoms from the last double bond to the terminal methyl group. In the old literature it was designated 18:2w6.
18-6=12, 12-3=9, hence D9,12.
The International Commission on Biochemical Nomenclature agreed to the first form of this nomenclature because of its interest in describing the fatty acid metabolism.
A list of common (non-systematic) names for fatty acids (by Adlof R.O. and Gunstone F.D.) with their structure and source may be consulted in a page from AOCS. The common names and the structures of many fatty acids may be found on the Lipidomics Gateway.
An important database available on Internet is the Lipid Bank for Web, a lipid database information retrieval system. It contains a lot of information about fatty acids and other lipid compounds. Additionally to physical and chemical data the database comprises information about the fatty acid composition of different oils.
The most documented fatty acids data collection is that of the Institute for Chemistry and Physics of Lipids in Münster growing since 1970. This electronically searchable Database SOFA (Seed Oil Fatty Acids, http://sofa.mri.bund.de/) offers a variety of search routines to browse into about 110,000 individual data relating to more than 7,000 different plant species (Aitzetmüller K et al., Eur J Lipid Sci Technol 2003, 105, 92). About 500 different fatty acids are listed. The database allows to search for plant species, genera and families, for individual fatty acids (start by adding an asterisk after each entry) and combinations of fatty acids in their seed oils, and for their percentage contents. It contains literature references and numerous unpublished data. Moreover, fatty acid partial structures or functional groups can also be searched for, using the "delta-notation" system of chemists as described above. The use of the database is mostly straightforward and self-explanatory but several examples for search operations have been published to help anybody interested in seed oils and their fatty acid composition (Aitzetmüller K et al., Eur J Lipid Sci Technol 2003, 105, 92).
A comprehensive database of melting points of fatty acids, collected from the literature and from original measurements has been reported (Guendouzi A et al., Chem Phys Lipids 2012, 165, 1-6). A newly developed methodology for the fast selection of descriptors in quantitative structureproperty relationships analysis of 62 fatty acids was also proposed.
A graphical chart of the oxidative degradation of fatty acids may be found on the Slideshare web site or the Medical Biochemistry Page.
Fatty acids can be subdivided into well-defined families
according to their chain structure and then to their other functional group :
A – Normal fatty acids (straight chain)
AA – Carbon chain without substituent
1 – Saturated fatty acids
2 – Monoenoic fatty acids
3 – Polyenoic fatty acidsMethylene-interrupted
Polymethylene-interrupted
Conjugated
Allenic acids
Cumulenic acidsAB – Carbon chain with substituent (other than methyl)
5 – Sulfur containing fatty acids
7 – Methoxy and acetoxy fatty acids
8 – Keto fatty acids
10 – Halogenated fatty acids (F, Cl, Br)
11 – Nitrated fatty acids
B – Branched-chain fatty acids
1 – Mono or multibranched chain fatty acids
2 – Branched methoxy fatty acids
3 – Branched hydroxy fatty acids (Mycolic acids)
C – Ring containing fatty acids
2 – Cyclobutane acids (ladderanes)
4 – Furanoid acids
5 – Cyclohexyl and hexenyl acids
6 – Phenyl and benzoic alkanoic acids
7 – Epoxy acids
9 – Lipoic acid
D – Fatty acyl-CoA
They have commonly straight chains and even carbon number (4-30). They have the general formula: CH3(CH2)nCOOH
They are named from from the saturated hydrocarbon with the same number of carbon atoms, the final -e is changed to -oic. For example, the fatty acid with 18 carbon atoms is correctly termed octadecanoic acid but it has also a trivial name (as several common fatty acids), i.e. stearic acid. This compound may be defined also 18:0.
Below, is found a list of the most common saturated fatty acids.
|
Systematic name |
Trivial name |
Shorthand designation |
Molecular wt. |
Melting point (°C) |
| propanoic | propionic | 3:0 | 74.1 | -20.5 |
| butanoic | butyric | 4:0 | 88.1 | -7.9 |
| pentanoic | valeric | 5:0 | 102.1 | -34.5 |
| hexanoic | caproic | 6:0 | 116.1 | -3.4 |
| heptanoic | oenanthic | 7:0 | 130.1 | -7.5 |
| octanoic | caprylic | 8:0 | 144.2 | 16.7 |
| nonanoic | pelargonic | 9:0 | 158.2 | 12.5 |
| decanoic | capric | 10:0 | 172.3 | 31.6 |
| undecanoic | undecylic | 11:0 | 186.3 | 29.3 |
| dodecanoic | lauric | 12:0 | 200.3 | 44.2 |
| tetradecanoic | myristic | 14:0 | 228.4 | 53.9 |
| pentadecanoic | 15:0 | 242.4 | 51-53 | |
| hexadecanoic | palmitic | 16:0 | 256.4 | 63.1 |
| heptadecanoic | margaric (daturic) | 17:0 | 270.4 | 61.3 |
| octadecanoic | stearic | 18:0 | 284.4 | 69.6 |
| eicosanoic | arachidic | 20:0 | 312.5 | 75.3 |
| docosanoic | behenic | 22:0 | 340.5 | 79.9 |
| tetracosanoic | lignoceric | 24:0 | 368.6 | 84.2 |
| hexacosanoic | cerotic | 26:0 | 396.7 | 88 |
| heptacosanoic | carboceric | 27:0 | 410.7 | |
| octacosanoic | montanic | 28:0 | 424.8 | |
| triacontanoic | melissic | 30:0 | 452.9 | |
| dotriacontanoic | lacceroic | 32:0 | 481 | |
| tritriacontanoic | ceromelissic (psyllic) | 33:0 | 495 | |
| tetratriacontanoic | geddic | 34:0 | 509.1 | |
| pentatriacontanoic | ceroplastic | 35:0 | 523.1 |
SOLUTION PROPERTIES
Normal fatty acids exhibit appreciable solubility in water compared to the corresponding hydrocarbons due to the presence of the polar carboxyl group. The first members of the saturated fatty acid series are miscible with water in all proportions at room temperature.
Solubility in water at 20°C (in grams acid per liter)
| Carbon number | Solubility |
| 2 | infinite |
| 3 | infinite |
| 4 | infinite |
| 6 | 9.7 |
| 8 | 0.7 |
| 10 | 0.15 |
| 12 | 0.055 |
| 14 | 0.02 |
| 16 | 0.007 |
| 18 | 0.003 |
The solubility behavior of the fatty acids in organic solvents is of considerable theoretical and industrial importance. Solubility data for the most common saturated fatty acids are given in the table below (in grams per liter at 20°C).
| Carbon number | Chloroform | Benzene | Cyclohexane | Acetone |
Ethanol 95% |
Acetic acid | Methanol | Acetonitrile |
| 10 | 3260 | 3980 | 3420 | 4070 | 4400 | 5670 | 5100 | 660 |
| 12 | 830 | 936 | 680 | 605 | 912 | 818 | 1200 | 76 |
| 14 | 325 | 292 | 215 | 159 | 189 | 102 | 173 | 18 |
| 16 | 151 | 73 | 65 | 53.8 | 49.3 | 21.4 | 37 | 4 |
| 18 | 60 | 24.6 | 24 | 15.4 | 11.3 | 1.2 | 1 | <1 |
On the basis of solubility data, it can be concluded that the normal saturated fatty acids are generally more soluble in chloroform and less soluble in acetonitrile than in any of the organic solvents investigated.
Up to 6 (or 4) carbon atoms, organic acids are considered "short-chain organic acids", they have substantial solubility in water. Furthermore, they do not behave physiologically like other fatty acids since they are more rapidly digested and absorbed in the intestinal tract and have unique properties in regulating sodium and water absorption through the mucosal epithelium. Biochemically, they are more closely related to carbohydrates than to fats.
From 7(or 6) to 12 carbon atoms, fatty acids are said to have a medium chain. Physiological studies have shown that ingestion of triglycerides containing these medium-chain fatty acids may result, as for short-chain fatty acids, in increased energy expenditure via faster satiety. Thus, they facilitate weight control when included in the diet as a replacement for long-chain triglycerides (St-Onge MP et al., J Nutr 2002, 132, 329).
Fatty acids which have 14 and more carbon atoms are considered as long-chain fatty acids.The specific physiological roles of saturated fatty acids have been reviewed (Legrand et al., Eur J Lipid Sci Technol 2015, 117, 1489).
Fatty acids with 4 to 12 carbon atoms are found mainly in milk fats (mainly butyric acid in cow and decanoic acid in sheep) but those with 10 and 12 carbon atoms are found also in certain seed oils such as coconut and other kernel fats of the palm family. Nevertheless, bacterial fermentation of amylase resistant starch and nonstarch polysaccharides in the gut is probably the most important source of short-chain fatty acids in humans
and most mammalian species.
It has been suggested that 15:0 and 17:0 from adipose tissue or plasma could be considered as potentiel biomarkers of milk products intake (Wolk A et al., J Nutr 2001, 131, 828). It has been shown that the same relationship can be established with saturated fatty acids (15:0, 17:0, 20:0) acylating lysophosphatidylcholine (Nestel PJ et al., Am J Clin Nutr 2014, 99, 46). However, these odd-chain fatty acids can also be synthesized endogenously, for example, from gut-derived propionic acid (3:0). A number of studies have also shown an inverse association between the concentrations of these compounds in human plasma phospholipids or erythrocytes and risk of type 2 diabetes and cardiovascular disease.
It was proposed a possible involvement in metabolic regulation from the assumption that there is a link between 15:0 and 17:0 and the metabolism of other short-chain, medium-chain, and longer-chain odd-chain fatty acids (Pfeuffer M et al., Adv Nutr 2016, 7, 730).
Some peculiarities of the metabolic features of short-chain and medium-chain fatty acids that differ from those of long-chain fatty acids are summed up in an important review (Schonfeld P et al., J Lipid Res 2016, 57, 943).
Valeric acid (5:0) has been identified in petroleum distillates and in oxidation products of oils and fats and fermentation of carbohydrates. It has a putrid odor.
Caproic acid (6:0) occurs in milk fats to the extent of about 2%. It was first isolated from butter in 1816 by Chevreul. It has a characteristic odor of goats, hence its name (from the Latin caper, goat). Caproic acid is present as glucose ester in leaf trichomes of Datura metel.
Caprylic acid (8:0) is widely distributed in animal and vegetable fats but rarely exceeding 8% of the total fatty acids, except in the seed oils of two Lythraceae, Cuphea hookerina and C. painteri, which contain about 70% caprylic acid (Miller RW et al., JAOCS 1964, 41, 279). It occurs to an extent of 1 to 4% in milk fats, and 6 to 8% in coconut and palm oils. Caprylic acid is a component of the active form of ghrelin, a 28 amino acids peptide produced by the mammalian stomach (Kojima M et al., Nature 1999, 402, 656). Ghrelin binds to the growth hormone secretagogue receptor (GHSR-1a) located in the pituitary gland and hypothalamus and regulates many relevant biological processes including the secretion of growth hormone (GH), stimulation of appetite, food intake and modulation of gastric acid secretion and motility (Delporte, C., Scientifica 2013, 2013, 518909).
As in ghrelin, octanoylation concerns the formation of an ester bond between caprylic acid and the side chain of a serine residue (Ezanno H et al., Nutr Clin Metabol 2013, 27, 10).
Pelargonic acid (9:0) is the first example of the occurrence of an odd-numbered carbon fatty acid in natural products. It occurs in secretion of sebaceous glands and in essential oil of Pelargonium roseum from which it derives its name. It is also a primary product of oxidative fission of oleic acid.
Capric acid (10:0) occurs as a minor component in the same fats that contain caprylic acid but also in the head oil of the sperm whale, and in wool and hair fats. It is a major constituent of elm seed oil (over 60% in Ulmus americana and over 70% in Zelkova serrata ) but is absent in other Ulmaceae (Apanthe, Morus) (Badami RC et al., Prog Lipid Res 1981, 19, 119). Similarly, it was discovered that the seed oil of a Lythraceae, Cuphea llavea, contained about 80% of this acid (Earle FR et al., JAOCS 1960, 37, 440).
Lauric acid (12:0) is one of the three most widely distributed saturated fatty acids found in nature (14:0, 16:0, and 18:0). It occurs extensively in Lauraceae seeds (Laurus nobilis) where it was discovered (Marsson T Ann 1842, 41, 329). It is dominant in cinnamon oil (80-90%), coconut oil (40-60% as trilaurin) and is found also in Cuphea species (Umbelliferae) whose production was initiated in Germany. The recent uses of lauric acid are in the manufacture of flavourings, cocoa butter, margarine, alkyd resins, soaps, shampoos and other surface active agents, including special lubricants. Lauric acid as monoglyceride is known to the pharmaceutical industry for its good antimicrobial properties. It may play a role in combating lipid-coated RNA and DNA viruses. The major sources of lauric acid for human food are palm kernel, coconut and palm.
Myristic acid (14:0) is present in major amounts in seeds of the family Myristicaceae (nutmeg oil – or oil of mace – from Myristica fragrans contains about 60-70% of trimyristin) where it was first discovered (Playfair L Ann 1841, 37, 152). Nutmeg is found in Moluccas and spice islands of Indonesia. Coconut and palm kernel are also convenient sources of 14:0 (trimyristine) which may be isolated in a pure form by distillation. It is also present in milk fats (8-12%) and in the head oil of the sperm whale (15%). An excess of myristic acid in the diet induces a rise in plasma cholesterol in animals and human being (Mensink RP et al., Arterioscler Thromb 1992, 12, 911). Among saturated fatty acids, only myristic acid is able to make an amide link with some cellular proteins (myristoylation), modification which regulates their biological activities (Johnson DR et al., Annu Rev Biochem 1994, 63, 869).
Palmitic acid (16:0) is the commonest saturated fatty acids in plant and animal lipids.

It was purified first by Chevreul in his researches on butter and tallow, but was first surely characterized by Fremy E (Ann 1840, 36, 44), who prepared it in pure form from palm oil, from which he named it. Despite its wide distribution, it is generally not present in fats in very large proportions. It usually forms less than 5% of the total fatty acids, sometimes as much as 10% in common vegetal oils (peanut, soybean, corn, coconut) and in marine-animal oils. Lard, tallow, cocoa butter palm oil contain 25 to 40% of this component.
As for myristoylation, palmitoylation (S-acylation) corresponds to the reversible attachment of palmitic acid to the side chain of a cysteine residue via a thioester bond.
Stearic acid (18:0) was described by Chevreul (1823) in the course of his researches on fats. It is the highest molecular weight saturated fatty acid occurring abundantly in fats and oils. It occurs in small quantities in seed and marine oils. Milk fats (5-15%), lard (10%), tallow (15-30%), cocoa and shea butters ((30-35%) are the richest sources of stearic acid. It is the principal constituent of hydrogenated fats and oils (about 90%).
The longer chains are less frequent, they can be found in uncommon seed oils (C20-24 in Leguminoseae and Sapindaceae), in palm oil (C20-C32)(Puah CW et al., Lipids 2006, 41, 305), in waxes (C24-30) and in some sphingolipids (C20-24). Long-chain saturated fatty acids (from C24 to C28) are produced by microalgae and it was estimated that diatoms contribute from 30 to 80% of these components in sandy sediments (Volkman JK et al., Org Geochem 1998, 29, 1163). These long-chain fatty acids derive from higher plant waxes and are more abundant in deep than in surface sediments (Rieley G et al., Org Geochem 1991, 17, 901; Muri G et al., Org Geochem 2004, 35, 1083).
Arachidic acid (20:0) occurs in appreciable quantities in groundnut (Arachis hypogea) oil (3%) where it was discovered in 1854 by Gössmann A (Ann Chemie 1854, 89, 1). Larger amounts are found in seeds of Sapindaceae (up to 20%). It is also found in the depot fat of some animals and in milk fats.
Behenic acid (22:0) was first reported as a constituent of ben (behen) oil (seeds of Moringa oleifera) (Voelcker A Ann 1848, 64, 342). Except for the seed oils of the Crucifereae (between 0.5 and 3.4%), this fatty chain does not occur in the principal oils. Large amounts are found in hydrogenated animal and vegetal oils (8-57%).
Lignoceric acid (24:0) is present at trace levels in plant oils except in groundnut oil (about 1%) and notably in a Leguminous seed oil (Adenanthera pavonina) where it may amount to about 25%. It is the principal fatty acid present in carnauba wax (30% of the normal fatty acids). A major source is rice-wax bran (about 40%).
Without double bonds or other functional groups, these fatty acids are nearly chemically inert and thus can be subjected to drastic chemical conditions (temperature, oxidation).
Saturated fatty acids were shown to be the major constituents of adipocere (similar to "adipocire" studied by Chevreul), the white and soap-like decomposition product which forms due to the post-mortem conversion of body adipose tissue (Pfeiffer S et al., J Forensic Sci 1998, 43, 368). Immediately following death, triglycerides are hydrolyzed into free fatty acids and glycerol. The free fatty acids (mainly myristic, palmitic, and stearic acids) present in characteristic relations (Forbes SL et al., For Sci Int 2002, 127, 225 ; Eur J Lipid Sci Technol 2003, 105, 761) are formed by hydrogenation of triglyceride components under suitable environmental conditions. During that conversion process a number of byproducts may be formed, such as hydroxy or keto fatty acid derivatives (Takatori T, For Sci Int 1996, 80, 49). The occurrence of salts of these saturated fatty acids has been suggested as resulting from reaction with the surrounding mineral environment.
Olfaction-based research have shown that carboxylic acids (from C4 to C16) play an essential role in the host-seeking behavior of the malaria mosquito
Anopheles gambiae (Smallegange RC et al., J Chem Ecol 2009, 35, 933).
Saturated fatty acids with straight chain have been found in a number of sediments ranging in age from Precambrian to Recent. In most sediments, fatty acids with even-carbon chain are more abundant than those with odd-carbon chain. All fatty acids from C8 to C28 have been found in sediments (Kvenvolden KA, JAOCS 1967, 44, 628). Experiments suggest that normal paraffins in petroleum may be produced from normal fatty acids of longer chain lengths by decarboxylation or other chemical reactions.
1 – Branched chain fatty acids are found most frequently with an unsubstituted carbon chain (some branched polyunsaturated fatty acids are found in sponges) but may have one or several branched methyl groups :
Mono or multibranched chain fatty acids
2 – Branched chain fatty acids (mono- branched) may have also a methoxy or a hydroxy substitution, they are found in exotic animals or bacteria :
Branched methoxy fatty acids
Branched hydroxy fatty acids (Mycolic acids)
Mono- and multibranched fatty acids
As for hydrocarbons, they have usually either an iso-structure (methyl group at the penultimate carbon atom) or a anteiso-structure (methyl group on the third carbon from the end).
Examples: 14-methyl pentadecanoic acid (isopalmitic) is of the iso-series and 13-methyl pentadecanoic acid is the corresponding anteiso-acid.
1 – Monomethyl branched fatty acids are found in vegetal, animal, and microbial lipids but in small concentrations. In animals, some classic examples of these compounds include the 2- and 4-monomethylated fatty acids from the uropygial gland of ducks (Kolattukudy PE et al., Arch Biochem Biophys 1991, 284, 201), as well as from the guinea pig Harderian gland (Yasugi E et al., J Biochem 1991, 110, 202). Iso– and anteiso-fatty acids from 8 to 30 carbons are present in high proportions in sebum (Nordstrom KM et al., J Invest Dermatol 1986, 86, 700) and in vernix caseosa (biofilm covering the human fetus) (Rissmann R et al., J Invest Dermatol 2006, 126, 1823).
In vegetals, 14-methyl-16:0 has been identified in Ginkgo biloba (Hierro MTG et al., J. Am Oil Chem Soc, 1996, 73, 575) and was found to be characteristic of pine seed oil (up to 1%). This fatty acid was found exclusively in Pinaceae (genera Pinus, Abies, Cedrus, Picea …) (Wolff RL et al., Lipids 1997, 32, 971). The unsaturated 11-methyloctadec-12-enoic and 12-methyloctadec-10-enoic acids were identified in the seed oil of Byrsocarpus coccineus (Connaraceae) (Spencer GF et al., Lipids 1979, 14, 72). A trans-monounsaturated branched-chain fatty acid, 8-methyl-trans-6-nonenoic acid, is characteristic of specific compounds, the capsaicinoids, found in fruits of the genus Capsicum (Solanaceae). These plants (bell pepper, chili pepper) are among the oldest cultivated plants, their pungent fruits being used as spices for over 6000 years. Capsaicinoids are synthesized by an enzymatic condensation of vanillylamine and a medium chain branched acid (Thiele R et al., J Agric Food Chem 2008, 56, 4219). More than 20 compounds, different only in the fatty acid structures, have been described.
The sponges contain also large quantities of C14 up to C30 fatty acids with branch as well as odd-chains (Carballeira N et al., Lipids 1989, 24, 229). As an example, a new structure (20-methyl-26:0) has been elucidated in the sponge Verongia aerophoba from the Canary islands (Nechev J et al., Eur J Lipid Sci Technol 2002, 104, 800) while some monomethyl polyunsaturated fatty acids were described in different marine sponges (24-methyl-5,9-pentacosadienoic acid or 2-methoxy-13-methyl-6-tetradecenoic acid) (Caballeira NM et al., J Nat Prod 2001, 64, 620) . Similarly, two new 2-methyl branched monoenoic very long chain fatty acids (2-methyl-24:1 n-7 and 2-methyl-26:1 n-9) were described in a marine sponge Halichondria panicea (Imbs AB et al., Chem Phys Lipids 2004, 129, 173). A new branched monounsaturated fatty acid (2-methyl-13-eicosanoic acid) has been described in a temperate clacisponge Leuconia johnstoni (Quevrain E et al., Lipids 2012, 47, 345).
It must be noticed that the similarity between the composition of the midchain branching pattern of fatty acids in some sponges and in bacteria suggests the presence of bacteria in these sponges. For Calcarea, the constant and prominent occurrence of iso– and anteiso-fatty acids (>40% of the total fatty acids in most species) suggests an origin of these compounds from the sponge cells rather than from bacterial lipids (Schreiber A et al., Chem Phys Lipids 2006, 143, 29). Another indication for a sponge cell origin of these compounds is the major presence (7-15 % of the total) of anteiso-nonadecanoic acid (16-methyloctadecanoic acid), an exotic compound that has not yet been reported as a major fatty acid in bacteria.
It has been found that Caenorhabditis elegans is able to synthesized iso-C15 and iso-C17 and that these branched-chain fatty acids are essential for the animal growth and development (Kniazeva M et al., PLoS Biol 2004, 2, E257). These results suggest that these fatty acids may play a potentially important role in other eukaryotes.
The occurrence of branched-chain fatty acids as major constituents in bacteria was first reported for Bacillus subtilis (Kaneda T, J Biol Chem 1963, 238, 1222). Branched-chain fatty acids of the iso and anteiso series occur widely in bacteria are of value in improving bacterial systematics. It should be noted that the fatty acid profile of bacteria with a branched-chain lipid type is affected by its growth conditions. The biosynthesis, function and taxonomic significance of branched-chain fatty acids in bacteria have been reviewed (Kaneda T, Microbiol Rev 1991, 55, 288).
A new unsaturated methyl-branched fatty acid, 9-methyl-16:1(n-6) and the uncommon 11-methyl- 18:1(n-6) were found in the lipid extract of a new strain of bacterium Vibrio alginolyticus associated with the alga Cladophora coelothrix (Carballeira NM et al., Lipids, 1997, 32, 1271). Another novel methyl-branched fatty acid, 10-methyl-18:1(n-9), was found in the lipid extract of the marine fungus Microsphaeropis olivacea (Yu CM et al., Can. J. Chem., 1996, 74, 730).
The diffusible factor, which regulates the virulence in Xanthomonas campestris, likely as in several other bacterial species, was identified as cis-11-methyl-2-dodecenoic acid (Wang LH et al., Mol Bacteriol 2004, 51, 903).

cis-11-methyl-2-dodecenoic acid
Unexpectedly, this key component of microbial cell-cell communication systems is also able, as farnesoic acid, to regulate the morphological transition and virulence in a fungal pathogen, Candida albicans.
Branched methyl-substituted fatty acids of bacterial origin are commonly found in lake or marine sediments, decreasing rapidly with depth (Matsuda H et al., Geochim Cosmochim 1977, 41, 777). Long-chain monomethyl-branched anteiso acids were also identified in settlings particles and surface sediments from freshwater lakes where they may be useful molecular markers for lake acidity (Fukushima K et al., Org Geochem 2005, 36, 311).
10-Methyl octadecanoic acid (tuberculostearic acid) is present at the sn-2 position in the phosphatidylinositol mannosides found mainly in several Mycobacterium species (Gilleron M et al., J Biol Chem 2001, 278, 29880). Later, a similar position of tuberculostearic has been described in phosphatidylethanolamines from Mycobacterium tuberculosis (Horst B et al., J Lipid Res 2010, 51, 1017). The detection of this fatty acid in cerebrospinal fluid was proposed as a possibility for rapid and specific diagnosis of tuberculous meningitis. The presence of this fatty acid in sputum lipids was successively utilized for the diagnosis of tuberculous pneumonia (Larsson L et al., J Clin Microbiol 1987, 25, 893). 10-methyl nonadecanoic acid (phytomonic acid) is also found in Mycobacterium.
In animals, 17-methyl-6-octadecenoic and 17-methyl-7-octadecenoic acids were identified in the Australian mollusk Siphonaria denticulata (Carballeira NM et al. J Nat Prod 2001, 64, 1426).
An unusual complex and polyunsaturated fatty acid substituted with one hydroxyl and one aldehyde group has been described as a new polyene pigment, laetiporic acid, in the wood-rotting basidiomycete Laetiporus sulphureus (Weber RW et al., Tetrahedron lett 2004, 45, 1075). This orange pigment, with an UV-visible spectrum similar to that of carotenoids, bears an unprecedented decaene skeleton as part of its chromophore.
2 – Multimethyl branched acids are found mainly in bacteria.
Several dimethylated fatty acids (14 or 16 carbon atoms) with the first methyl substituent at carbon 2 or 4 have been isolated from a halophilic Bacillus species (Carballeira NM et al., J Nat Prod 2001, 64, 256).
Di- and tri-methylated fatty acids with 15 to 18 carbon atoms have been isolated from environmental subsurface sediments (Hedrick DB et al., Lipids 2008, 43, 843). These fatty acids may be of value for the knowledge of biomass and of the metabolic status of the viable microbial community.
A novel dimethylated fatty acid, 12,17-dimethyloctadecanoic acid, has been described in high concentration (16.3 % of total fatty acids) in an extremophile bacteria, Thermogemmatispora sp. (Vyssotski M et al., Lipids 2012, 47, 601).
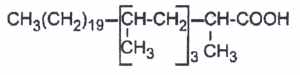
Multimethyl branched acids are abundant in cell wall lipids of Mycobacteria, each methyl group being on even carbon atoms (2,4,6,8…from the methyl end). Thus, forming waxes and glycolipids (mycosides), several multibranched fatty acids are commonly found :
– mycolipanolic acid (2,4,6-trimethyltetracosanoic acid). It has been isolated from the lipids of human tubercle bacilli (Coles L et al., J Chem Soc 1968, 1541-4).
– mycoceranic acid(2,4,6-trimethyloctacosanoic)
– mycolipenic acid (2,4,6-trimethyl-trans-2-tetracosenoic acid)
– micolipodienoic acid (2,4,6-trimethyltetracosadienoic acid)
– mycocerosic acid (2,4,6,8-tetramethyl C32 fatty acid)
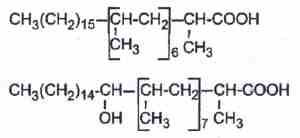
– phthioceranic acids which are hepta or octamethyl fatty acids, some of them being also hydroxylated (hydroxyphthioceranic acid).
– dolichoic acids have been discovered is lipid extracts of human brain (Ward W et al., J Lipid Res 2007, 48, 1457). These acids, derived from dolichols, contain 14-20 isoprene units. Future work remains in determining the biosynthetic pathway for these acids and elucidating their function within the brain.
The analysis of mycolic, mycocerosic and mycolipenic acids and phthiocerol compounds in old skeletons from a human settlement at Atlit-Yam, Israel (~9,000 year-old) and from an extinct Bison antiquus from Natural Trap Cave, Wyoming (~17,000 year-old) have provided evidence for tuberculosis in these landmark specimens (Lee OY et al., Tuberculosis 2015 S1472-9792). The hypothesis that this disease evolved as a zoonosis, before transfer to humans has been previously discussed (Lee OY et al., PLoS One. 2012;7(7):e41923).
Branched acids but with shorter chains are also found in the depot fats of ruminant animals, in sebum and animal waxes (wool-wax). Ruminants produced a huge variety of C10-C18 acids with one to four branched methyl groups, they were detected in lamb adipose tissue. Multimethyl branched acids are also dominant in the uropygial waxes of the birds preen gland. Curiously, they were also identified in the lipid depot in the head of marine animals, location involved in their echo-locating abilities.
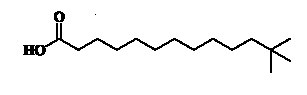
Unusual branched fatty acids have been isolated as minor components from the glycolipids (GL) fraction of freshwater sponges (Dembitsky VM et al., Chem Phys Lipids 2003, 123, 117).It is possible that these neo acids could be of cyanobacterial origin. An example of one of them with the longest carbon chain is shown below.
Others have isoprenoid structures, thus coming from the diterpene phytol derived from chlorophyll and not by the de novo pathway. Among them, two are found in marine organisms, in geological sediments but, one of them (phytanic acid or 3,7,11,15-tetramethyl hexadecanoic acid) is present in human diet or in animal tissues where it may be derived from chlorophyll in plant extracts. Phytanic acid is present in cow milk fat but its concentration is affected by feed composition (Che BN et al., J Agric Food Comp 2013, 61, 225).
Two very unusual phytyl esters were obtained from the extract of the hornwort Megaceros flagellaris (Bryophyte, Anthocerothae). The fatty acid moiety comprises 3,7,11,15-tetramethyl-16:1 or 3,7,11,15-tetramethyl-16:0, which is esterified to the corresponding tetramethyl unsaturated (16:1) alcohol (Buchanan MS et al., Phytochemistry, 1996, 41, 1373).
Phytanic acid derives from the corresponding alcohol, phytol, and is oxidized into pristanic acid.
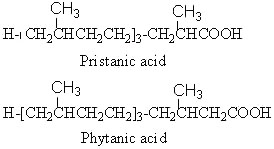
Pristanic acid was first isolated from butter (Hansen RP et al., Biochemical Journal 1964, 93, 225). The name of the substance is derived from pristane (2,6,10,14-tetramethylpentadecane), the corresponding hydrocarbon which was isolated from shark (pristis in Latin). It is also found in the lipids from many sources such as sponges, crustacea, milk fats, animal depot fat but also in petroleum samples.
Phytanic acid characterizes a precise human pathology, the Refsum’s syndrome. This inherited neurological disorder (Refsum S, Acta Psychiat Scand Suppl 1946, 38, 9) is characterized by a accumulation of phytanic acid, normal metabolite of phytol, in blood and tissues (Klenk E et al., Hoppe Seyler’s Z Physiol Chem 1963, 333, 133). The disorder was later related to deficiency in the alpha-oxidation pathway in the liver (Herndon JH et al., J Clin Invest 1969, 48, 1017; review in : Mukherji M et al., Prog Lipid Res 2003, 42, 359-376). Both phytanic acid and pristanic acid have been shown to activate the peroxisome proliferator-activated receptor ![]() (PPAR
(PPAR![]() ) in a concentration-dependent manner (Zomer AW et al., J Lipid Res 2000, 41, 1801). The presence of phytanic acid in food is related to the fatty alcohol phytol, contained in chlorophyll, ingested by ruminants and fish. The bioconversion of phytol into phytanic acid is effective in the rumen and in the marine environment. Thus, milk, other dairy products and meat from ruminants as well as fish contain the highest concentrations of phytanic acid in the range of 100-500 mg/100 g lipids. Persons suffering from the Refsum syndrome must restrict to the minimum the absorption of dairy products and ruminant flesh.
) in a concentration-dependent manner (Zomer AW et al., J Lipid Res 2000, 41, 1801). The presence of phytanic acid in food is related to the fatty alcohol phytol, contained in chlorophyll, ingested by ruminants and fish. The bioconversion of phytol into phytanic acid is effective in the rumen and in the marine environment. Thus, milk, other dairy products and meat from ruminants as well as fish contain the highest concentrations of phytanic acid in the range of 100-500 mg/100 g lipids. Persons suffering from the Refsum syndrome must restrict to the minimum the absorption of dairy products and ruminant flesh.
Freshwater sponges contain polymethyl branched fatty acids such as 4,8,12-trimethyltridecanoic, phytanic and pristanic acids. These acids may have chemotaxonomical significance for both marine and freshwater sponges (review in : Dembitsky VM et al., Chem Phys Lipids 2003, 123, 117). The isoprenoic 4,8,12-trimethyltridecanoic was found to be always present in the marine calcareous sponges (Calcarea) but in minor amounts (Schreiber A et al., Chem Phys Lipids 2006, 143, 29), this acid being presumed to be derived from phytol, a degradation product of chlorophyll. Phytanic acid present in immature (recent) sediments is thought to derive from phytol, a supposition backed by stereochemical studies (Maclean I et al., Nature 1968, 218, 1019).
Few sources, including sponges, contain branched polyunsaturated fatty acids (Rezanka T, Prog Lipid Res 1989, 28, 147). As an example, freshwater Demospongia (Spongillidae) were shown to contain di-, tri-, and tetramethyl substituted dienoic, tetraenoic, and hexaenoic fatty acids (Rezanka T et al., J Nat Prod 2002, 65, 709). A review of these rare polyenoic fatty acids was released by Dembitsky VM et al (Chem Phys Lipids 2003, 123, 117).
Some isoprene derivatives (sesquiterpenes) synthesized by invertebrates from farnesoic acid have important endocrinological functions (juvenile hormones) such as molting, reproduction and metamorphosis.
A lactone derivative of a terpenoid fatty acid hydroxylated on the carbon 3 (Vittatalactone) has been determined as a pheromone emitted by the male striped cucumber beetles, Acalymma vittatum, an insect which causes a major damage to cucurbit crops in North America (Morris BD et al., J Nat Prod 2005, 68, 26).
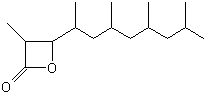
Vittatalactone
Addition of isoprene acids to proteins (prenylation), discovered in 1984 (Schmidt RA et al., J Biol Chem 1984, 259, 10175), is an important post-translational modification of proteins which has been recognized as a key physiological process for facilitating cellular protein-protein interactions and membrane-associated protein trafficking. Protein prenylation occurs by the covalent addition of two types of isoprenoids, farnesyl pyrophosphate (a 15-carbon sesquiterpene) or geranylgeranyl pyrophosphate (a 20-carbon diterpene), to cysteine residues at or near the terminal carboxyl group . The largest family of prenylated proteins are the intracellular GTP-binding proteins that transduce extracellular signals into intracellular changes via downstream effectors (McTaggart SJ, Cell Mol Life Sci 2006, 63, 255).
Some isoprenoid fatty acids with conjugated double bonds are known. In this group, the most interesting is retinoic acid which derives from retinol but is synthetized ultimately from b-carotene (provitamin A). It has important functions in cell regulation.
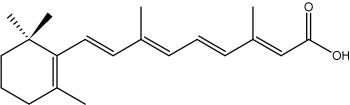
all-trans-Retinoic acid
Abscissic acid, a methylated derivative of retinoic acid, plays a variety of roles in plant physiology. It is ubiquitous in plants, including algae.
Iso or anteiso alpha-methoxylated C15-C16 fatty acids were reported in phospholipids of a Caribean sponge Amphimedon complanata (Carballeira NM et al. Lipids 2001, 36, 83).
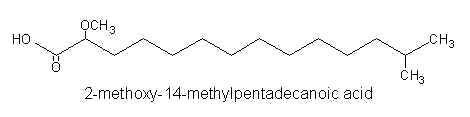
Thus, 2-methoxy-13-methyltetradecanoic acid, 2-methoxy-14-methylpentadecanoic acid, and 2-methoxy-13-methyl pentadecanoic acid were identified by gas-liquid chromatography and mass spectrometry.
It has been suggested that these compounds have originated from a novel bacteria in symbiosis with the sponge.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire