
STEROIDS
Steroids form an important group of compounds based on the fundamental saturated tetracyclic hydrocarbon : 1,2-cyclopentanoperhydrophenanthrene (sterane or gonane).
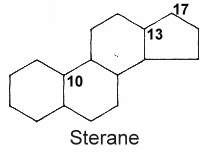
This nucleus, partially or completely hydrogenated, is generally substituted by methyl groups at C10 and C13. A chemical group (ketone, hydroxyl…) or an alkyl side-chain may also be present at C17. Steroids may possess a nucleus derived from sterane by one or more C-C bond scissions or ring expansions or contractions.
The term "steroids" was coined by Callow RK et al. (Proc Royal Soc London series A 1936, 157, 194) "for the group of compounds comprising the sterols, bile acids, heart poisons, saponins, and sex hormones".
As natural steroids are derived from squalene by cyclization, unsaturation and substitution, they may be considered as modified triterpenes. Fatty acid esters of steroids are found mainly in the blood but their exact role is not known to date. An efficient analytical method for the simultaneous determination of 12 esters in serum has been desscribed (Jung HJ et al., J Chromatogr A 2009, 1216, 1463).
There is a close connection between modern-day biosynthesis of particular triterpenoid biomarkers and presence of molecular oxygen in the environment. Thus, the detection of steroid and triterpenoid hydrocarbons far back in Earth history has been used to infer the antiquity of oxygenic photosynthesis (Summons RE et al., Phil Trans R Soc B 2006, 361, 951).
According to their chemical structure, the wide array of steroid molecules may be divided into several groups :
Sterols
Brassinosteroids
Bufadienolides
Cardenolides
Cucurbitacins
Ecdysteroids
Sapogenins
Steroid alkaloids
Withasteroids
Bile acids
Hormonal steroids
They are derivatives of cholestane with two vicinal diols (C-2, C-3 and C-22, C-23) and a 6-keto group.
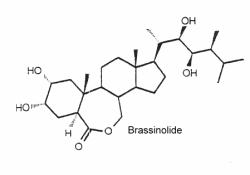
They are a unique class of plant growth regulators with structural similarity to animal steroid hormones, and ecdysteroids from insects and crustacea. Many of them may be considered as sterols. The first biologically active compound isolated from the pollen of Brassica napus in 1979 is brassinolide (Grove MD et al., Nature 1979, 281, 216). Over 60 analog compounds have been isolated but brassinolide exhibits the highest biological activity of the known brassinosteroids.
As it was shown that these compounds could be potent plant growth and development regulators, dozens of compounds of similar structure were isolated from plant sources (algae, ferns, gymnosperms, and angiosperms, but not bacteria) or synthesized. It was shown that they interact with jasmonates in the formation of anti-herbivory traits in tomato (Campos ML et al., J Exp Bot 2009, 60, 4347).
Extensive reviews on brassinosteroids released by Zullo MAT and Clouse SD may be consulted with interest. The complex role of brassinosteroids in plant developmental and physiological responses has been reviewed (Gudesblat GE et al., Curr Opinion Plant Biol 2011, 14, 530). It has been demonstrated that brassinosteroids also confers freezing tolerance in plants (Eremina M et al., PNAS 2016, 113, E5982).They are typically polyhydroxy C24 steroids with a pentadienolide ring at C-17. The structure of hellebrigenin is given below as a typical example of bufadienolides.
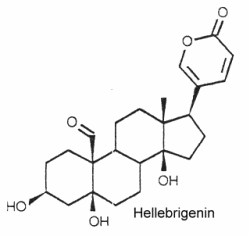
They have been isolated from plants and animals. More than 250 compounds have been identified. In plants, thay are mostly glycosides with one to three sugars in a chain linked to the 3-hydroxyl group.
They are important for their cardiotonic activity. Furthermore, they possess insecticidal and antimicrobial properties, those produced by the toad skin are strongly poisonous.
An extensive review on bufadienolides released by Steyn may be consulted with interest.
Their structure is closely related to bufadienolides but these C23 steroids possess a butenolide ring located at C-17. The structure of digitoxigenin is given below as a typical example of cardenolides.
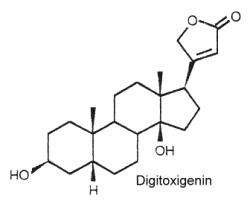
They are widely distributed in plants mainly as glycosides and are either toxic or insect deterrents. As potent cardiotonics, through their inhibition of Na/K- ATPases, these steroids were largely studied (digoxin and its derivative ouabain…). Monarch butterfly is well known to be highly toxic to birds because of cardenolides which come from the milkweed leaves eaten by its caterpillar. Experimentally, the larvae of the lepidopteran Trichoplusia ni were poisoned by feeding on the milkweed Asclepias curassavica, which contains cardenolides in its latex (Dussourd DE et al. Chemoecology 2000, 10, 11).
They are the most oxygenated C30 triterpenoids with a dimethyl group at C-4 and methylated at C-9 and C-14. Strictly, they are not steroid since they are not methylated at C-10. The structure of cucurbitacin D is shown below as a typical example of cucurbitacins.
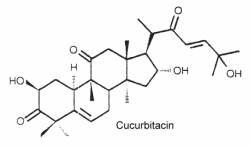
These steroids which are commonly combined in glycosides, are mainly associated with cucurbitaceae species but they have also been detected in other families. About 50 species have been identified.
Mammals perceived these toxic molecules as some of the bitterest substances known. They have protective effects against herbivores but are feeding stimulants for some beetles. Cucurbitacins have been shown to act as ecdysteroid receptor antagonists.
These C27 steroids have in common a 7-en-6-one chromophore, sometimes a methyl group at C-24 and several hydroxyl groups increasing their polarity. The first ecdysteroid was isolated as a molting hormone (ecdysone) in 1954 by Butenandt and Karlson. The structure of ecdysone is given below as an example of these steroids. Its structure was first elucidated from a hormonal fraction extracted from silk worm pupae (Karlson P, Naturwissenschaften 1966, 53, 445).
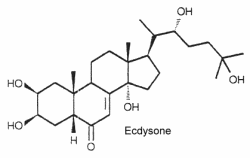
Ecdysteroids are present both in animals (arthropods) and plants. About 400 species have been identified.
In plants, they are named "phytoecdysteroids" and they seem to protect plants against most insects. Most phytoecdysteroids possess a cholest-7-en-6-one carbon skeleton and a hydroxyl group on the C14. The carbon number can vary between C19-C29 (sometimes C30). The most common phytoecdysteroid in plants is ecdysone (20Ehydroxyecdysone). Among many structures, we noticed the presence of ajugalactone jn Ajuga reptans (Labiatae), ajugasterone C in Vitex madiensis (Verbenaceae), cyasterone in Ajuga chamaepitys (Labiatae), inokosterone in Achyranthes fauriei (Amaranthaceae), makisterone B in Ajuga chamaepitys (Labiatae), ponasterone A in Podocarpus nakaii (Podocarpaceae), polypodine B in Polypodium vulgare (Polypodiaceae) and poststerone in Cyathula capitata (Amaranthaceae). Insects that ingest phytoecdysteroids and are not adapted to this defense are subject to serious adverse effects, including reduced weight, molting disruption, and/or mortality.
In insects, precursors are produced by prothoracic glands and metabolites are known to trigger a cascade of morphological changes through specific receptors (molting hormones). The most efficient is 20-hydroxyecdysone. Relationships between plants and insects have been hypothesized. It appears that all arthropods employ essentially the same compound as the molting hormone.
An extensive data base may be consulted with interest.
They form the aglycon part of saponins which have well known detergent properties. They are oxygenated C27 steroids with an hydroxyl group in C-3. The structure of diosgenin is given below as an example of these compounds.
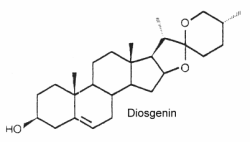
These steroids can mimic or regulate steroid hormones. Thus, diosgenin can be chemically converted into corticosteroids, estrogens and progesterone. They are externally distributed in plants. They are extremely distributed in plants since they occur in over 90 plant families. They are used in nutrition, as herbal medicine, and in cosmetics.
They form a large group of molecules where a nitrogen atom is integrated into a ring or in a substituent. The steroid nucleus can contain double bonds and hydroxyls in various positions. The structure of solasodine is given below as an example of these compounds.
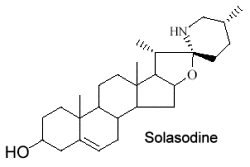
These alkaloids are only distributed in Solanaceae (potato, tomato, eggplant …). Fortunately, their toxic properties disappear by structural transformation during ripening. Solasodine is the most common species in Solanum.
They typically C28 ergostane-type steroids with a 22,26-lactone.They are also characterized by a large number of oxygenated functions (hydroxyls, ketones, epoxides …). 90% of withasteroids (or withanolides) possess a 1-oxo-group as shown below in withaferin.
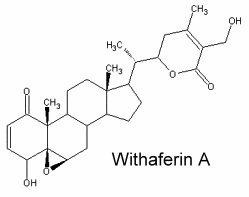
Over than 200 species are known, some of them as glycosides. They are predominantly associated with Solanaceae but are also found in other families (Taccacceae, Leguminosae, Labiatae). Withanolides are known to have important pharmacological properties (anti-tumor, immunosuppressive) but they are also antimicrobial, insect deterrent or ecdysteroid receptor antagonists.
The end products of cholesterol utilization are the bile acids, synthesized in the liver. In mammals, the most common bile acids are C24 steroids with a carboxyl group at C-24 and up to three hydroxyl groups on the steroid nucleus, one being at C-3. The most abundant bile acids in human bile are chenodeoxycholic acid (45%) and cholic acid (31%). These are referred to as the primary bile acids. Within the intestines the primary bile acids are converted by bacteria into the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). These compounds are reabsorbed by the intestines and delivered back to the liver via the portal circulation. Within the liver the carboxyl group of primary and secondary bile acids is conjugated via an amide bond to either glycine or taurine before their secretion into the bile. These conjugation reactions yield glycoconjugates and tauroconjugates, respectively. They are hydrolized in the intestine. Glycoconjugates are present among eukaryotes only in mammals, but they were also detected (with deoxycholic acid) in a marine bacterium, Myroides sp. (Maneerat S et al., Appl Microbiol Biotechnol 2005, 67, 679).
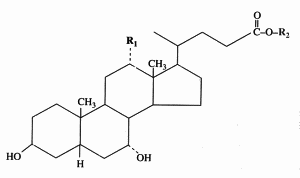
Cholic acid : R1 = OH, R2 = H
Chenodeoxycholic acid : R1 = R2 = H
Glycocholic acid : R1 = OH, R2 = NH-CH2-COOH
Taurocholic acid : R1 = OH, R2 = NH-CH2-CH2-SO3H
Bile acids have long been known to be essential in dietary lipid absorption and cholesterol catabolism. Furthermore, an important role for bile acids as signaling molecules has emerged. They were shown to activate mitogen-activated protein kinase pathways, to be ligands for the G-protein-coupled receptor TGR5 and to activate nuclear hormone receptors (Houten SM et al., EMBO J 2006, 25, 1419). Bile acids have been discovered to activate specific nuclear receptors (FXR, PXR, Vitamin D receptor), a cell surface receptor (TGR5), and cell signaling pathways (JNK 1/2, AKT and ERK 1/2) in cells in the liver and gastrointestinal tract (review in Hylemon PB et al., J Lipid Res 2009, 50, 1509). Several works provide evidences of bile acid signaling in regulation of glucose and lipid metabolism (Li T et al., J Lipids 2012, ID 754067).
Several 3-keto-cholestenoic acids (dafachronic acids) were shown to be involved in the control of dauer formation and reproduction in the nematode Caenorhabditis elegans (Motola DL et al., Cell 2006, 124, 1209). Investigations revealed that the nuclear hormone receptor DAF-12 from that worm was optimally activated by two isomers, (25S)-D7- and (25S)-D4-dafachronic acid with EC50 values of 23 and 33 nM, respectively (Sharma KK et al., Mol Endocrinol 2009, 23, 640). One of these isomers is shown below.
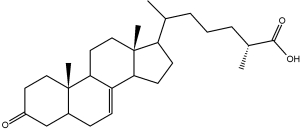
(25S)-D7-Dafachronic acid
It has been shown that bile acids were able to function as nutrient signaling molecules primarily during the feed/fast cycle as there is a flux of these molecules returning from the intestines to the liver following a meal
induce. They regulate the increase in energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin (Watanabe M et al., Nature 2006, 439, 484). This novel metabolic effect is dependent on induction of the cyclic-AMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase.
It was shown in Caenorhabditis elegans that a dietary restriction or starvation leads to an increased production of that steroid that is also required, but not sufficient, for the diet-mediated longevity enhancement (Thondamal M et al., Nat Commun 2014, 5:4879).
A simple and rapid procedure for the isolation of bile acid fraction using a solid-phase extraction on a C18 column has been described (Persson E et al., J Lipid Res 2007, 48, 242).
Conjugates of fatty acid with bile acids are a new class of molecules synthesized with the aim of reducing cholesterol crystallization in bile. Among them, arachidyl amido cholic acid (Aramchol) was shown to be the most active to retard that process and may be of potential use in cholesterol gall stone disease in humans (Gilat T et al., Gut 2001, 48, 75).
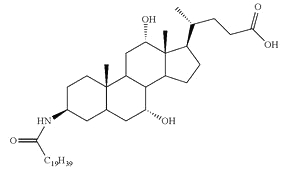
Aramchol : Arachidyl amido cholic acid
Aramchol has been shown to activate cholesterol efflux by strongly stimulating the ABCA1 transporter, a universal cholesterol export pump present in all cells (Leikin-Frenkel A et al., Arch Med Res 2010, 41, 6). In animal models, this led to a significant reduction of blood and body cholesterol and an increase in fecal sterol output, mostly neutral sterols.
A documented review on bile acids, present in all vertebrates except fish and bile alcohol present in fish, may be consulted (Hofmann AF et al., Cell Mol Life Sci 2008, 65, 2461).
Bile acids determination : As early as 1974, an almost complete separation of bile acids was done by TLC (Fausa O et al., Scand J Gastroenterol 1974, 9, 567). Analyses using HPLC coupled with refractive index detector or with ultraviolet detection were proposed, but their low sensitivity limited their applications. More recently, it has been shown that evaporative light-scattering detection improved both selectivity and sensitivity (Persson E et al., J Lipid Res 2007, 48, 242). A further improvement was observed using an isocratic HPLC charged aerosol detector which enabled the determination of individual bile acids in human gastric and duodenal aspirates (Vertzoni M et al., J Lipid Res 2008, 49, 2690).
This large group can be divided into three major families, mainly on the basis of their physiological function or their tissue origin : the sexual hormones, the corticosteroids, and the neurosteroids.
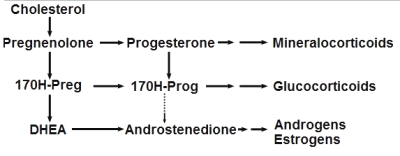
Steroidogenesis pathway
(from Wikipedia Commons)
While best known in vertebrates, the presence of several steroids in aquatic invertebrates (echinoderms, molluscs, and crustaceans) have been already reported (Janer G et al., Ecotoxicology 2007, 16, 145). However, these results obtained by immunoassay methods should be regarded with caution, because of possible cross-reactivity and interferences (Devier MH et al., Anal Chim Acta 2010, 657, 28).
Pregnane is the parent of progesterone, ketones, and several adrenocortical hormones. It is found largely in urine as a metabolic product of 5ß-pregnane compounds. It is found also in ancien sediments and petroleum.
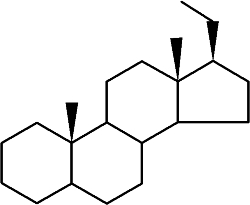
Pregnane
While the abundance of pregnane isomers relative to regular steranes increases in oil with increasing thermal maturity, this was attributed to differences in thermal stability. It was suggested that pregnane compounds originate from kerogen-bound sterol precursors during diagenesis and catagenesis (Wang G et al., Org Geochem 2015, 78, 110). They represent novel indicators in exploration of ancient sediments and petroleum.
This important group may be again divided into estrogens, progestagens, and androgens. It must be noticed that androstenedione, produced in the adrenal glands and the gonads (ovary and testicles), is the common precursor of all sexual hormones (see Steroidogenesis pathway). In females, androstenedione is produced by theca cells and exported in granulosa cells for estrogen production. This steroid has a fuunction of sexual pheromone in fish (Sorensen PW et al., Gen Comp Endocrinol 2005, 140, 164).
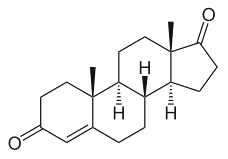
Andostenedione
This compound was also detected in the pollen of a pine species (Pinus sylvestris) (Saden-Krehula M. et al., Experientia 1971, 27, 108). It has been also reported in Nicotiana tabacum and Inula helenium at a level of 8 to 11 pmol/g (Simersky R et al., J Plant Growth Regul 2009, 28, 125). Its presence is also documented in waters and bottom sediments of rivers which receive paper mill effluent.
Estrogens : They are C18 steroids generally with a phenolic function at C-3 (the first ring A being aromatic), without methyl group at C-10, and with always an oxygenated function at C-17. 17b-estradiol is the model molecule.
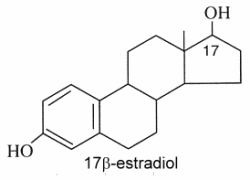
Estrone (or folliculin) is a compound similar to 17b-estradiol but with a ketone group on the C-17. Secreted by the ovary, it has estrogenic activity but is also present in plants (pollen and seeds of date palm, seeds of pomegranate). This hormone was discovered by Butenandt A (Nobel Prize in chemistry, 1939).
The glucuronide derivative of estradiol has been found in high concentrations in seawater around spawning Euphyllia ancora (a stony coral) seawater, and has therefore been implicated as a candidate signaling molecule in spawn synchronisation (Twan WH, et al., Fish Physiol Biochem, 2005, 31, 111). It seems thus evident that Corals already evolved the vertebrate-type hormone system in their sexual reproduction.
Progestagens : They are C21 steroids with a en-4-one-3 group and a ketone function at C-20. Progesterone is the model molecule. In 1934, Butenandt A (Nobel Prize in chemistry, 1939) and Westphal U succeeded in producing this hormone in a chemically pure form.
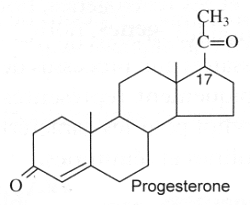
High levels of progesterone have been reported in plants, Nicotiana tabacum and Digitalis purpurea (55 to 59 pmol/g) (Simersky R et al., J Plant Growth Regul 2009, 28, 125).
Guggulsterone is an analogue of progesterone. That sterol is found in the resin of the guggul tree (Commiphora mukul).
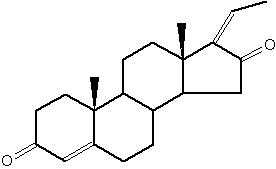
Guggulsterone
Guggul tree extract has been suggested to lower low-density lipoprotein levels in animal models, it has been successfully used in Ayurveda medicine since at least 600 BC to treat obesity and lipid disorders (Satyavati GV et al., Indian J Med Res 1988, 87, 327; Singh V et al., Pharmacol Res 1990, 22, 37). It has been also shown that guggulsterone is an efficacious antagonist of the liver X receptor (FXR) and the bile acid receptor (Urizar NL et al., Science 2002, 296, 1703); Wu J et al., Mol Endocrinol 2002, 16, 1590). It has been proposed that inhibition of FXR activation is the basis for the cholesterol-lowering activity of guggulsterone.
Sulfate and ester derivatives of guggulsterone have been proposed as component of nanosomes or liposomes for drug delivery (Ahmad MU et al., Chem Phys Lipids 2010, 163, 362).
Androgens : They are C19 steroids. The major androgen is testosterone which is a 17b-hydroxysteroid with a en-4-one-3 group. This steroid was also detected in the pollen of Scots pine (Pinus sylvestris).
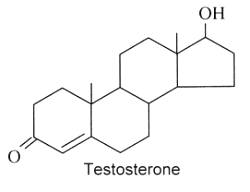
Several testosterone derivatives are present in human male secretions such as sweat, saliva, and semen and have been implicated as putative human pheromones. Among them, the most studied is androstadienone (Grosser BI et al., Psychoneuroendocrinology 2000, 25, 289). It was shown that smelling androstadienone was able to maintain higher levels of cortisol in women (Wyart C et al., J Neurosci 2007, 27, 1261).
Androstenol and androstenone were also characterized in human sebum.
|
|
|
|
Theoretically, these compounds, named also corticoids, should be formed in the adrenal cortex. Furthermore, they must be C21 steroids and three or more oxygen atoms. They have all a en-4-one-3 group and an oxygenated function at C-20. The major corticosteroids in vertebrates are cortisol which has an hydroxyl group at C-11, C-17, and C-21 (glucocorticoid hormone) and aldosterone which has only one hydroxyl group at C-11 and one aldehyde function at C-18 (mineralocorticoid hormone).
|
|
|
Corticosteroid hormones in vertebrates are critical for metabolism, growth, reproduction, immunity, and ion homeostasis, and are an important part of the coping mechanisms involved in the stress responses. In tetrapod groups, there are at least two active glucocorticoid hormones, either cortisol or corticosterone, and one mineralocorticoid hormone, aldosterone, which regulates ion balance. In contrast, in teleosts, cortisol apparently has both activities, whereas aldosterone is not present. It has been clearly determined that 11-deoxycortisol (one of the precursors of cortisol) is the only corticosteroid hormone present in the earliest vertebrates, the agnathans (the lamprey) (Close DA et al., PNAS 2010, 107, 13942).
Recent discoveries have revealed that brain is a site of extensive steroid metabolism and also a target of steroid hormones. These hormones play an important role in the development, growth, maturation and differentiation of the brain (Baulieu EE, Psychoneuroendocrinol 1998, 23, 963). The term "neurosteroid", proposed by EE Baulieu in 1981, applies to steroids which are accumulated in the brain independently of supply by peripheral endocrine glands and which are synthesized from cholesterol in the nervous system. Several steroids have been described in the brain since the first report in 1981 of dehydoepiandrosterone (DHEA) and its sulfated derivative in the rat brain (Corpechot C et al., PNAS 1981, 78, 4704). Among the best known are pregnenolone, progesterone, allopregnanolone and DHEA. A review of the pleiotropic and protective abilities of neurosteroids and hormonal steroids may be consulted (Melcangi RC et al., Cell Mol Life Sci 2008, 65, 777).
Pregnenolone is the product of cholesterol conversion by a P450 oxydase complex (cholesterol side-chain cleavage enzyme, P450scc) and is the immediate precursor of progesterone. This steroid is found in free form or as a sulfated derivative. Its function is mainly a negative modulation of GAGA-A receptor activity and a positive modulation of NMDA receptors. Several studies suggest that pregnenolone plays an important role in the control of neural development and in the improvement of neuron plasticity.
|
|
Progesterone, as described above is a progestagen, but also is active at the brain level. That important steroid is formed directly from pregnenolone in neurons and glial cells by a 3b-hydroxysteroid dehydrogenase. Its sedative and anesthetic properties have been described as soon as 1941 by H. Selye. Among its numerous functions, it can be noticed that progesterone has important consequences for myelinisation, neuronal development, survival and regeneration of the nervous system.
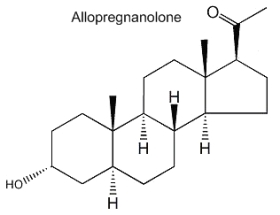
Allopregnanolone (3a-hydroxy-5a-pregnan-20-one) is formed from progesterone by the action of 5a-reductase and 3b-hydroxysteroid dehydrogenase. It acts mainly in modulating the GABA-A receptor activity and its physiological role is important in neurogenesis, survival and migration of neurons. Furthermore, its involvement in the stress reaction suggests that this neurosteroid could have implications in depressive disorders.
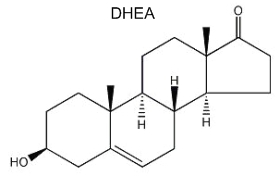
Dehydroepiandrosterone (DHEA) is the first neurosteroid discovered in a mammalian brain and, thus, is the most actively studied. DHEA is a direct metabolite of pregnenolone and is found in free form or as a sulfated derivative. The great interest in this neurosteroid is the observation of its abundance in human brain and blood and its concentration lowering during stress situations and aging. Recent studies amphasize its role in neurogenesis, survival and protection of neuronal cells.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire