
These compounds form the simplest form of lipids, they contain only carbon and hydrogen. They may be divided into aliphatic hydrocarbons with a carbon chain which may be linear (normal), branched, saturated (alkanes) or unsaturated (alcenes), cyclic hydrocarbons and carotenoids. Several hydrocarbons may be substituted with oxygen-containing groups.
These organic compounds include :
These molecules are found mainly in petroleum but living organisms, eukaryotic or prokaryotic, contain frequently hydrocarbons which are directly derived from fatty acids. They are known to be present in living matter since 1892 when Shall C (Chem Ber 1892, 25, 1489) identified undecane in ants, and Etard A (C R Acad Sci Paris 1892, 114, 364) identified eicosane in Bryonia dioica. They are distinct from the terpenoid hydrocarbons. They have usually a straight chain of up to about 36 carbon atoms but may also be branched, with one or more methyl groups attached at almost any point of the chain. Usually, the methyl group is near the end of the chain (iso or anteiso). They are either saturated or unsaturated (mono or diunsaturated). In contrast with the diversity of methyl-branched alkanes found in insect species, n-alkanes predominate in plants. Among the least polar components of plant surface lipids hydrocarbons with the odd number carbon chains (C15 up to C33) are predominant.
Allenic hydrocarbons, such as 9,10-tricosadiene, 9,10-pentacosadiene, and 9,10-heptacosadiene were isolated from Australian insects (melolonthine scarab beetles) (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
Many microalgae contain the highly unsaturated alkene n-C21:6 formed by decarboxylation of the 22:6n-3 fatty acid (Lee RF et al., Phytochemistry 1971, 10, 593). A few species also contain the n-C21:5 alkene (Volkman et al., Org Geochem 1994, 21, 407). Several microalgae were shown to contain long-chain unsaturated alkenes from 19 to 38 carbon atoms and one to four double bonds (review in Volkman JK et al., Org Geochem 1998, 29, 1163).
Hydrocarbons are found at the outer surface in higher plant leaves. As an example, C27, C29, and C31 n-alkanes are the most abundant (from 11 to 19%) in needle wax of the Pinaceae Picea omorika (Nikolic B et al., Chem Nat Compounds 2009, 45, 697). Environmental parameters such as temperature and humidity can affect the composition of higher plant leaf hydrocarbon wax. The abundance and distribution of long-chain n-alkanes in sediments have been proposed as chemical indicators reflecting climate change. Thus, it has been shown that aridity specifically affected the concentration and distribution of n-alkanes in Acacia and Eucalyptus sampled in the North of Australia. Their n-alkane concentration increased by a factor of ten from the sea to the dry center of Australia, but Acacia-alkanes decreased in average chain length towards the arid center of Australia, whereas Eucalyptus average chain length increased under arid conditions. (Hoffmann B et al., Org Geochem 2013, 62, 62).
Field bioassays enabled the discovery of alkanes as bee sex pheromones and orchid attractants. Thus, C21 to C27 alkanes elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37).
They are also abundant at the outer surface of insects and several marine organism. They are thought to serve as a barrier to water influx in the organism, to act as sex attractants (or anti-aphrodisiacs), to affect the absorption of chemicals and microorganisms. Wild populations of Drosophila melanogaster use several cuticular hydrocarbons (mainly 7,11-heptacosene) as sexual pheromone (Cobb M et al., Anim Behav 1990, 39, 1058). The roles of hydrocarbons in the recognition systems of insects has been reviewed (Singer TL, Amer Zool 1998, 38, 394). The blend of linear and branched hydrocarbons from 22 to 34 carbon atoms found on the cuticle of various species of the Coleoptera genus Chrysochus has been shown to mediate mate choice and sexual isolation (Petereson MA et al., Chemoecology 2007, 17, 87). The 7-methyltricosane is a male-predominant cuticular hydrocarbon used as a contact pheromone in the western flower thrips Frankliniella occidentalis (Thysanoptera) (Olaniran OA et al., J Chem Ecol 2013, 39, 559).
Several hydrocarbons are produced as alarm pheromones by the Dufour’s gland in the ants (Regnier F E et al., J Insect Physiol 1968, 14, 955). Five hydrocarbons have been described, undecane, tridecane, pentadecane, 2-tridecanone, 2-pentadecanone. During the act of stinging, formic acid and hydrocarbons are discharged simultaneously from these glands in fine droplets. These hydrocarbons act also as spreading agents for formic acid. 9-Tricosene (23 C) is found in the web of some male spiders (Pholcidae) (Schulz S, J Chem Ecol 2013, 39, 1)
Cuticular hydrocarbons have proved to be useful for identifying insect species and differentiating populations. In combination with cuticular hydrocarbons, isoprenoid soldier defensive secretions have been used in some termite species for chemotaxonomic analyses. Thus, analyses have shown that the hydrocarbon profiles of French populations of subterranean termites, Reticulitermes flavipes, were closer to termite populations from Louisiana than to those from Florida (Perdereau E et al., J Chem Ecol 2010, 36, 1189). In ants (Formica exsecta), the chemical profile has been extensively studied across Europe, demonstrating a hydrocarbon profile, composed mainly of homologous series dominated by three n-alkanes (C23, C25, and C27) and three (Z)-9-alkenes (C23:1, C25:1, and C27:1) (Martin SJ et al., J Chem Ecol 2013, 39, 1415). Only the (Z)-9-alkenes have been shown to act as nest-mate recognition cues, with changes in n-alkanes corresponding with task differences. Similar results have been described in other ants and in honeybees. It has been shown that n-alkanes and (Z)-9-alkenes respond to environmental factors, but often in different ways, indicating that their production is controlled by different genetic pathways. Studies of phenotypic variations in various ant population support the primary role of (Z)-9-alkenes as recognition cues and that of n-alkanes, and other cuticular lipids, as anti-desiccants.
Field bioassays enabled the discovery of alkenes as bee sex pheromones and orchid attractants. Thus, (Z)-9, (Z)-11 and (Z)-12 alkenes (C25, C27, C29) elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37). Additive bioassays indicated the importance of the alkene double bond position for controlling pollinator preference.
Several hydrocarbons (octane, nonane, dodecane, hexadecane…) belong to aroma compounds which are found in environmental or food systems (see the website Flavornet).
Various hydrocarbons are present in the photosynthetic prokaryotes but in low concentrations. Most species have from 15 to 20 carbon atoms, heptadecane being by far the most predominant in all species. In some species, mono- or di-unsaturated chains (alkenes) were found, in others (Cyanobacteria) methyl-branched alkanes are present.
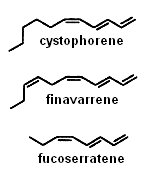
Several alkenes with 8 or 11 carbon atoms and 3 or 4 double bonds play a role in algae gamete attraction (pheromones) : cystophorene in Cystophora sp, finavarrene in Ascophyllum sp and Sphaerotrichia sp, fucoserratene in the brown seaweed Fucus serratus and in the freshwater diatom Asterionella formosa (Bacillariophyceae) .
Hydrocarbons are also formed as products of fatty acid cleavage during peroxidation processes. Alkanes as well as alkenes appear during hydroperoxide decomposition.
Chlorinated hydrocarbons with more than 15 carbons have been reported in the leaf waxes of halophytic Chenopodiaceae (Grossi V et al., Phytochemistry 2003, 63, 693). Occurrence of long chain chloroalkenes and chloroalkanes with 30 to 36 carbons have been reported in lake sediments from the Galapagos Islands (Zhang Z et al., Org Geochem 2013, 57, 1). The precursor of these long chain lipids remains yet an enigma.
Among hydrocarbons, a variety of forms are described (saturated or unsaturated):
The normal paraffins : their general formula is CH3(CH2)nCH3 (n= 6 – 40 or greater).
Paraffins may have branched chains :
– one methyl group (monobranched), iso-branched hydrocarbons (methyl group on the second carbon) or anteiso-branched hydrocarbons (methyl group on the third carbon)
(as below)
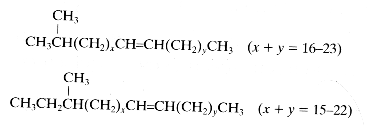
Among the almost 1,000 cuticular hydrocarbons present in ant species, about 200 monomethylakanes and 600 dimethylakanes are used for chemical communication (Martin S et al., J Chem Ecol 2009, 35,1151). Odd chain lengths and positions of methyl at odd carbon numbers are far more numerous than even chain-length compounds. That chemical recognition, fundamental in insect societies, is known for over 100 years in ants and was shown to be based on antennal detection of non-volatile compounds found on cuticle (Fielde AM, PNAS 1901, 53, 521). Since 1987, the nest-mate discrimination systems in several ant species are known to be based on hydrocarbons (Bonavita-Cougourdan et al., J Entomol Sci 1987, 22, 1).
In longhorned beetle, Mallodon dasystomus (Coleoptera, Cerambycidae), cuticular hydrocarbon profiles of females contained 13 compounds that were not present in profiles of males. Among the female-specific compounds, two co-dominant methylbranched alkanes, 2-methylhexacosane and 2- methyloctacosane, are contact pheromes and accounted for 17% of the total hydrocarbons (Spikes AE et al., J Chem Ecol 2010, 36, 943).
– several methyl groups (multibranched), one for each unit deriving from the isoprene formula : CH2=C(CH3)-CH=CH2. Hydrocarbons formed of isoprene units belong to the large group of terpenes.
This chain type is frequently found in several lipid forms, either isolated or combined with other chemical structures. A series of long-chain methylated alkanes (more than 23 carbon atoms), saturated or with one double bond, were identified in settling particles and surface sediments from Japanese lakes and were shown to be produced by planktonic bacteria being thus useful molecular markers (Fukushima K et al., Org Geochem 2005, 36, 311). Laboratory experiments have demonstrated that n-alkanes up to C35 may be formed in the laboratory under hydrothermal conditions (Fischer-Tropsch-type reactions) from formic acid or oxalic acid (Mccollum TM et al., Orig Life Evol Biosph 1999, 29, 153). These results support the theory of the origin of life in hydrothermal systems.
Methoxyalkanes have been identified on bodies or silk of spiders : 1-methoxy-16,20,24,28-tetramethylhentriacontane and 1-methyl-2,24-dimethyloctacosane (Schulz S, J Chem Ecol 2013, 39, 1).
It must be noticed that highly branched and unsaturated (2-5 double bonds) isoprenoids are widespread components in marine sediments (review by Rowland SJ et al., Marine Envir Res 1990, 30, 191; Belt ST et al., Geochim Cosmochim Acta 2000, 64, 3839). The identification of C25 and even of C30 highly branched isoprenoid alkenes in diatoms (Johns L et al., Org Geochem 1999, 30, 1471) have clearly established that they are the source of these compounds found in sediments.
Among the saturated isoprenoids found in geological sediments and oils, the most frequent are pristane (2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-tetramethylhexadecane). Both compounds can be generated diagenetically from the phytol side chain of chlorophyll. Pristane may also derive from the side chain of tocopherols while phytane is also generated by Archaea.
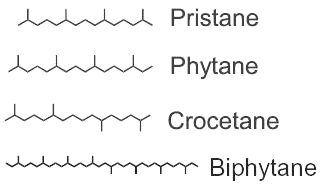
The widespread use of pristane as a biological marker is related to its structural similarity to phytol and its apparent stability, in connection with inability of microorganisms to carry out its anaerobic destruction. pristane is present in photosynthetic organisms, it has been detected in bacteria, algae, and higher plants. Marine sources of pristane include zooplankton, lobster, fish, sharks, sperm whale. Fossil fuels such as coal and petroleum contain this compound. The stable structure persists even in Precambrian rocks and perhaps in extraterrestrial meteorites. The coexistence of microfossils with pristane and phytane in Precambrian rocks is significant to the paleobotanist. Despite this inertness, pristane can be utilized as the sole source of carbon and energy for growth of a coryneform soil isolate ( McKenna EJ et al., PNAS 1971, 68, 1552).
Crocetane is formed by four isoprene units arranged symmetrically around a tail-to-tail linkage (2,6,11,15-tetramethylhexadecane). It was initially synthesized and named by Karrer et al. (Helv Chim Acta 1930, 13, 707). Crocetane is mainly present in oils and in methane-rich sediments, as it was shown to be produced by microorganisms utilizing methane as their carbon source. 2,6,10,15,19-Pentamethylicosane differs from crocetane by the addition of a single isoprene unit, joined head to tail, at one end of the molecule. This compound is present in methane-rich sediment and is likely produced by anaerobic methanotrophs.
Phytane and biphytane, present in sediments and petroleum, are thought to derive from ether-lipids (archaeol, caldarchaeol) of Archaea, the only organisms known to possess such structures.
The separation of straight chain and branched chain alkanes is efficient on a micro scale using the urea complex formation as described for fatty acids (Xu S et al., Org Geochem 2005, 36, 1334). That efficient method is based on the urea inclusion directly on the TLC plates and successive elutions with two solvents to separate straight and branched alkanes.
One important member of isoprenoid polyenes is squalene (C30H50) which is a metabolic precursor of sterols and steroids and classified into the triterpenoids. It is also a component of sebaceous lipids (12-15% of sebum weight) found on human skin. It consists of 6 isoprene units and contains 6 trans double bonds. It was discovered in 1906 in shark oil (Tsujimoto M, J Soc Chem Ind Jpn, 1906, 9, 953). It was suggested that squalene and its peroxidized derivatives (6 are possible) occurring by UV irradiation have an important role in the occurrence of sunburn, and/or protection from sunburn skin damage (Ohsawa K et al., J Toxicol Sci 1984, 9, 151). Furthermore, it has been suggested that squalene peroxides may play an important part in the pathology of acne, pityriasis versicolor, and skin aging. There is some evidence that squalene reduces colon cancer (Rao et al., Carcinogenesis 1998, 19, 287) and skin cancer (Owen R et al., Food Chem Toxicol 2000, 38, 647). This activity is likely related to its antioxidant effect (Amarowicz R, Eur J Lipid Sci Technol 2009, 111, 411).
![]()
It must be mentioned that if squalene is found in large quantities (from 0.2 to 0.9 g per Kg) in some fish liver oils (shark), it is also found in olive oil (its content may vary from 1 to 40 mg per Kg) where it is used to detect any adulteration. Other vegetable oils contain only traces of this compound (from 0.02 up to 0.3 mg per Kg). Squalene was also found in the epicuticular wax of fruit (grapefruit) and in the hydrocarbon fraction of wheat.
Squalene, and its saturated derivative (squalane) present in skin sebum, are largely used in cosmetics. Squalane is obtained by hydrogenation of the squalene pool isolated mainly from olive oil.
It has been shown that presqualene diphosphate, intermediate between farnesyl diphosphate and squalene, carries biological activity in human neutrophiles and serves as a negative intracellular signal preventing superoxide anion generation (Levy BD et al., Nature 1997, 389, 985). An inhibition of phosphatidylinositol 3-kinase in the same cells was also demonstrated (Bonnans C et al., J Exp Med 2006, 17, 203, 857). During that signaling step, squalene diphosphate is transformed into the inactive monophosphate species (Fukunaga K et al., J Biol Chem 2006, 281, 9490).
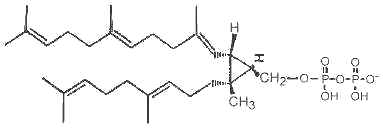 Presqualene diphosphate
Presqualene diphosphate
Two homologous series of trimethylalkanes, the 3,7,11-trimethylalkanes (C34H7), C36H74 and C38H78) and the 4,8,12-trimethylalkanes (C35H72, C37H76 and C39H80) have been described as the major constituents of the cuticular alkanes of the ant, Atta colombica (Martin MM et al., Tetrahedron 1970, 26, 307). Each of these structures combines a reduced polyketomethylene chain and a modified isoprenoid chain, and hence combine structural units from two major metabolic pathways. Hydrocarbons of this type have not been previously isolated from natural sources.
Pheromones with an epoxy ring are biosynthesized from unsaturated hydrocarbons by action of epoxidases specific for one specific double bond. In the case of monoepoxides derived from 3,6,9-trienes, all three kinds of epoxydienes (3,4-epoxy- 6,9-dienes, 6,7-epoxy-3,9-dienes, and 9,10-epoxy-3,6-dienes) have been found in female moths, 9,10-epoxy-1,3,6-triene has been identified as a natural pheromone component from two species in Arctiidae, the fall webworm (Hyphantria cunea) (Tóth M et al., Tetrahedron Lett 1989, 30, 3405) and the mulberry tiger moth (Lemyra imparilis) (Ando T et al., Topics Current Chem 2004, 239, 51). 9,10-Epoxy-(3Z,6Z)-1,3,6-henicosatriene has been identified from a pheromone gland of arctiid species, such as Hyphantria cunea (Yamakawa R et al., J Chem Ecol 2012, 38, 1042).
Hydrocarbons may be classified into monocyclic and polycyclic species.
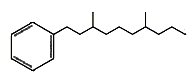
Monocyclic hydrocarbons : Several branched-alkylbenzenes have been described in Archaebacteria such as Thermoplasma and Sulfolobus. They have mostly two methyl groups branched on a saturated chain of 9 to 12 carbon atoms. One of them is shown below.
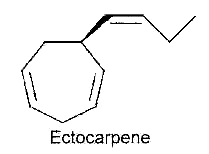
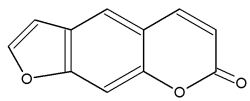
After the first hypothesis of a communication system (chemotaxis) by Thuret MG for the fertilization of brown algae, 117 years were needed to know the structure of the first algal pheromone (Müller DG et al., Science 1971, 171, 815). This compound, ectocarpene, is an unsaturated heptacyclic hydrocarbon found in the brown algae Ectocarpus, Adenocystis and Sphacelaria.
Several C11 alkylbenzenes similar to ectocarpene play a role of pheromone in marine brown algae, dityotene in Dictyota sp, desmarestene in Desmarestia sp and Cladostephus sp, lamoxirene in Laminaria sp, Alaria sp and many others.
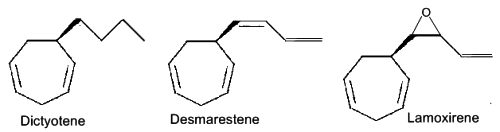
Alkylbenzenes from brown algae
The C11-hydrocarbon ectocarpene was also detected as component in the odor of ripening mango (Berger RG et al., J Agric Food Chem 1985, 33, 232) but was also shown to be a metabolite in leaves of the Asteraceae Senecio isatideus (Bohlmann F et al., Phytochemistry 1979, 18, 79). Several biosynthetic studies suggest unsaturated fatty acids as precursors of these pheromone hydrocarbons (Pohnert G et al., Nat Prod Rep 2002, 19, 108). A review of hydrocarbon as chemical signals in algal gamete attraction has been released (Boland W, PNAS 1995, 92, 37).
Several parent molecules (linear, tri-, penta- or hexacyclic) with different degrees of unsaturation and chain lengths have been described in various algal species (Pohnert G et al., Nat Prod Rep 2002, 19, 108).
A benzyl cyanide derivative has been described in the butterfly Pieris brassicae ( Andersson J et al., J Chem Ecol 2003, 29, 1489). It functions as an antiaphrodisiac transferred by the male to the female.
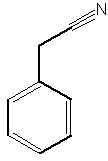
Benzyl cyanide
The stilbenes (or stilbenoids) form a group of numerous compounds based on a simple one, stilbene. That compound consists of two phenyl groups linked by a trans ethene double bond. Its name was derived from the Greek word "stilbos", which means shining. Stilbene is mainly used in manufacture of dyes and optical brightening agents.
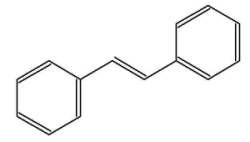
trans-stilbene
Stilbenes, sometimes classed into the polyphenol group, are present in several vegetal sources. Several forms have been described, differentiated by various substitutions and combinations of hydroxyl or alkoxyl groups. Some stilbenes are glycosylated. Thus, 2,3,5,4′-tetrahydroxystilbene 2-O-b-D-glucoside, the major bioactive compound from Polygonum multiforum, can efficiently inhibit the formation of advanced glycation end products (Lv L et al., J Agric Food Chem 2010, 58, 2239).
Resveratrol (3,4′,5-trihydroxystilbene) is the most studied because of its presence in grapes and wine and some berries (blueberries, Vaccinium) and its numerous pharmacological properties (anti-cancer, antiviral, neuroprotective, anti-aging, and anti-inflammatory).
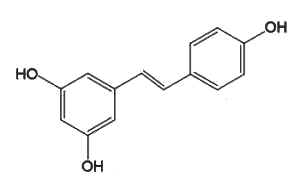
Resveratrol
Piceatannol, an analogue and metabolite of resveratrol (4 OH groups instead of 3), is a natural stilbene commonly found in grape skins and wine. Compared to resveratrol, this molecule exhibits superior bioactivities as an inhibitor of COX-1 and 2. Piceatannol is thought to be a potent natural compound with many therapeutic effects, such as the prevention of hypercholesterolemia, arrhythmia, atherosclerosis, angiogenesis, and cardiovascular diseases. It also demonstrates vasorelaxation, antioxidant, and anticancer activities (Seyed M.A. et al., J Agric Food Chem 2016, 64, 725).
Pterostilbene is a methylated derivative (the two hydroxyl group on the left cycle) of resveratrol. Based on animal studies and cultured human cancer cells, it displays anti-cancer (Mak KK et al., Mol Nutr Food Res 2013, 57, 1123), anti-hypercholesterolemia, anti-hypertriglyceridemia properties, and may have also the ability to fight against cognitive decline. This derivative is prfesent in grape and in blueberries (Rimando AM et al., J Agric Food Chem 2013, 52, 4713).
It has been determined that resveratrol is used by plants as a defensive element. Thus, grape vine attacked by
mildew is able to product resveratrol which can be transformed into glycosylated or dimer compounds. The resistance of some cultivars seems to be related to the toxicity of the derivative (Pezet R et al., Physiol Mol Plant Pathol 2004, 65, 269). Similar observations were reported for conifers.
Pharmaceutic industries have developed stilbene derivatives which have estrogenic activity (non-steroidal estrogens) such as diethyl stilbestrol.
Diarylheptanoids belong to a compound group having phenyl rings at 1,7 positions of n-heptane, such as curcumin and several similar analogues found in the rhizomes of the ginger (Curcuma longa) family (Li J et al., J Nat Prod 2010, 73, 1667). Their common structure is shown below.
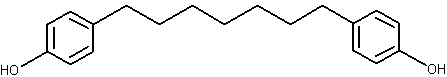
Diarylheptanoid
Curcumin is the principal diarylheptanoid of the Indian spice turmeric, which is a member of the ginger family (Zingiberaceae).
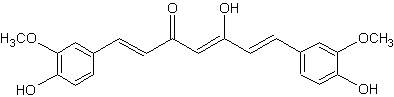
Curcumin
That pigment, which gives the yellow color to turmeric (E100), was isolated two centuries ago, and its structure as diferuloylmethane was determined in 1910. Numerous therapeutic activities have been assigned to turmeric for a wide variety of diseases and conditions, they are likely partly related to its strong antioxidant properties (Aggarwal BB et al., Adv Exp Med Biol 2007, 595, 1).
Plants of the Alnus genus (Betulaceae) have been found to be a valuable source of diarylheptanoids similar to curcumin but with various substituted chemical groups. Some of these compounds have valuable activities against LPS-induced inflammation and could be the source of new anti-inflammatory drugs (Lai YC et al., Phytochemistry 2012, 73, 84).
Polycyclic hydrocarbons : these hydrocarbons, whose only few groups are present in plants, may contain fused rings containing only carbon or heterocycles including foreign atoms such as oxygen, nitrogen, or several others.
– Polycyclic hydrocarbons containing only carbon :
Naphthalene is a constituent of Magnolia flowers (Azuma H et al., Phytochemistry 1996, 42, 999). It may function as protection of tissue against chewing insects and it may attract insects to pollinate by the UV absorption of accumulated naphthalene in the floral parts and floral scent.
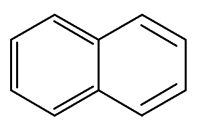
Naphthalene
Naphthoquinones are present in the secretion of scent glands of Opiliones (Raspotnig G et al., J Chem Ecol 2010, 36, 158). The two main components which serve chemical defense in these animals are 1,4-naphthoquinone and its methylated derivative 6-methyl-1,4-naphthoquinone.
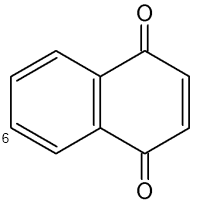
1,4-Naphthoquinone
Binaphthyl compounds have been isolated from the fungus (Ascomycetes) Daldinia concentrica (Hashimoto T et al., Chem Pharm Bull 1994, 42, 1528). One of them is shown below.
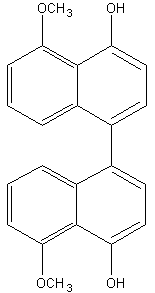
Perylene is a typical polyaromatic hydrocarbon that occurs in many sediments. Possible precursors include binaphthyl compounds derived from fungi, as those cited above, in which case a high abundance of sedimentary perylene might indicate a moist and humid continental climate in the depositional environment (Suzuki N et al., Org Geochem 2010, 41, 234).
Perylene
Several forms of phenanthrenes are present in in higher plants, mainly in Orchidaceae family but also in Dioscoreaceae, Combretaceae, Euphorbiaceae, Juncaceae and Hepaticae. The original phenanthrene molecule may be substituted in various positions by hydroxyl, methoxyl, methyl and/or prenyl groups. Most of them are monomeric but some dimeric and trimeric forms were described. As an example, the compound below (denthyrsinin) is reported in the orchid species Cymbidium pendulum, Dendrobium spp, Eulophia nuda, Nidema boothii, Scaphyglottis livida, Thunia alba. That compound, as others, displayed potent cytotoxic activities (review in Kovacs et al., Phytochemistry 2008, 69, 1084).
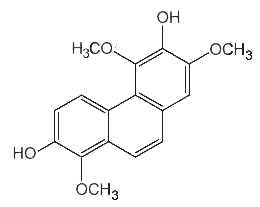
Denthyrsinin
New 9,10-dihydrophenanthrenes and phenanthrenes were isolated from Juncus setchuensis, a plant which has long been regarded as an antipyretic and detumescence agent in traditional Chinese medicine (Wang XY et al., J Nat Prod 2009, 72, 1209). Some of these compounds have shown strong antitumor and antialgal activities.
Many plants producing phenanthrenes are used in traditional medicine, likely in connection with the cytotoxicity, anti-microbial, spasmolytic, anti-allergic, anti-inflammatory activities of the natural phenanthrenes present in these plants.
The anthracene nucleus is present in several compounds detected in plants used in traditional medicine. Thus, anthraquinone is found in several plant species (Aloes), fungi and lichens but also in insects where they play a role of pheromone.
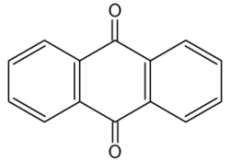
Anthraquinone
Chrysophanol is a member of the anthraquinone family. Pharmaceutical studies have shown that it exerts a number of biological effects, including anticancer and antimicrobial. The mechanism underlying the anti-inflammatory effects of chrysophanol is likely through the inhibition of caspase-1(Kim SJ et al., Molecules 2010, 15, 6436).
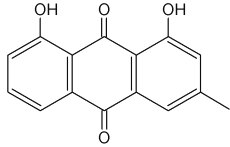
Chrysophanol
Although not included in the living world but obviously derived by combustion of plants, many polycyclic aromatic hydrocarbons (PAHs), such as methylphenanthrene (3 cycles), triphenylene and chrysene (4 cycles), benzopyrene (5 cycles) and the coronene (6 cycles) have been identified in sediments dating from the early Triassic period to more recent times. Retene (1-methyl-7-isopropyl phenanthrene) derives here from the diagenesis of compounds which were abundantly produced by the early Palaeozoic bryophytes in the upper Silurian-lower Devonian period (Romero-Sarmiento MF et al., Org Geochem 2010, 41, 302).
Some have a structure of terpenes, they are discussed in another chapter. Many others are outside the scope of this work because they obviously come from contamination by petroleum products or their derivatives.
Coumarins : the simplest compound of this group is coumarin. Several others are coumarin derivatives by various additions.
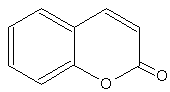
Coumarin
It is found in many plants, notably in the tonka bean from a tropical tree of the Fabaceae family (Dipteryx odorata). It is produced also by the Poaceae vanilla grass (Anthoxanthum odoratum) and buffalo grass (Hierochloe odorata), and by a Rubiaceae plant, woodruff (Galium odoratum). All these plants are strongly scented due to the presence of coumarin which has been used in perfumes since 1882 (imitation of vanilla products). Coumarin, used as rodenticide, and extracts from these plants are potential harmful as coumarin is the precursor for several anticoagulants, notably warfarin.
Some coumarin derivatives (phenylpropanoids) are present in various plants. Among them, umbelliferone, aesculetin, herniarin, psoralen and neoflavones. Scopoletin (6-methoxy-7-hydroxycoumarin) is a phytoalexin produced by several Solanaceae, such as tobacco (Nicotania tabacum), which protects plants against virus, bacteria or fungi (Oirdi ME et al., Environ Microbiol 2010, 12, 239).
Several coumarins with short- or long-chain hydrophobic groups have been described in roots of Angelica dahurica, a well-known traditional Chinese medicine (Wei W et al., Phytochemistry 2016, 123, 58), some of them having potent anti-inflammatory properties.
– Osthole [7-methoxy-8-(3-methylpent-2-enyl)coumarin] is a coumarin derivative found in Cnidium monnieri, a plant used in traditional Chinese medicine to treat skin affections. Osthole was shown to exhibit several biological functions, including antiosteoporotic, antiallergic, anti-inflammatory, and antitumor functions. Recently, it was found that osthole might be a potent antidiabetic agent (Lee W.H. et al., J Agric Food Chem 2011, 59, 12874).
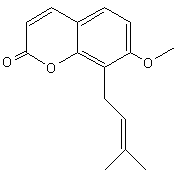
Osthole
– Umbelliferone (or 7-hydroxycoumarin) occurs in many familiar plants from the Umbelliferae family such as carrot or coriander but also from other families such the Asteraceae Pilosella officinarum. Umbelliferone absorbs ultraviolet light strongly but despite possible harmful mutagenic properties, is used in sunscreens. An umbelliferone methoxylated derivative, herniarin (7-methoxycoumarin) occurs in the leaves of tha Asteraceae water hemp (Eupatorium ayapana) and in several Herniaria (Caryophyllaceae).
– Psoralen (or psoralene) is a furanocoumarin. It is a derivative from umbelliferone by addition of a furan ring. Psoralen has been described in the seeds of the Fabaceae Psoralea corylifolia, but is present in many plants such as several Rutaceae (Ruta, Citrus), Moraceae, Leguminoseae Psoralea, Coronilla) and Apiaceae. Psoralen-rich plants are used in Chinese and Indian medicines and psoralene, due to its UV absorption properties, is used in treatment of psoriasis, eczema, vitiligo and in some cutaneous lymphoma. Psoralen is used in tanning accelerators, but it should be kept in mind that it increases the skins sensitivity to light.
Psoralene
Bergapten is a methoxylated derivative of psoralen (on carbon 5). It is found in bergamot essential oil and in other citrus essential oils. As it has a high phototoxicity, bergapten-free bergamot oil is now used in perfumery.
Many furanocoumarins are toxic and are produced by plants as a defense mechanism against various types of predators and in human, some of them (bergamottin) are responsible for the "grapefruit juice effect", in which they affect drug metabolism. Bergamottin is present in the juice but is more concentrated in the essential oil of bergamot. It is a linear furanocoumarins functionalized with a side chain derived from geraniol on the carbon 5 of the central ring. Bergamottin and dihydroxylated derivatives have been found to inhibit the human intestinal cytochrome P450 3A4 isozyme (CYP3A4) involved in the metabolism of some prescribed medications (Edwards DJ et al., Drug Metab Dispos 1996, 24, 1287).
They are also bactericide, fungicide, antiviral and insecticide and in plant they have an important role as inhibitor of germination.
Butenolides are modified hydrocarbons with a hetocyclic nucleus of the furan type. The simplest one is 2-furanone.
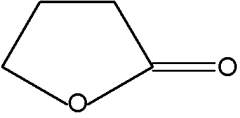
2-Furanone
That compound is characteristic of aroma of Apiacaea (genus Angelica). It can also inhibit the quorum sensing in bacteria. Several analogues or derivatives of 2-furanone are present in fruit aroma : 4-methoxy-2,5-dimethyl-3-furanone in pineapple and 4-hydroxy-2,5-dimethyl-3-furanone (furaneol) in strawberry and tomato. Furaneol is present in roasted peanut and tobacco smoke and 3,5,5-trimethyl-2-furanone appears in roasted in hazel-nut. Some other derivatives are active as sexual pheromones in insects.
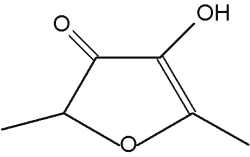
Furaneol
Several derivatives of 2-furanone with a polyacetylenic side chain have been isolated from Asteraceae (Vernonia scorpioides) and displayed antiherpeetic activities (Pollo LAE et al., Phytochemistry 2013, 95, 375).
A review of the naturally occuring furanones may be consulted (Slaughter JC et al., Biol Rev 1999, 74, 259)
Karrikins are a butenolide family of plant growth regulators found in smoke derived from burning plant material (mainly cellulose) (Flematti GR et al., Science 2004, 305, 977). These compounds are derived from 2-furanone by condensation with a pyrane group. They act as a key germination trigger for many species from fire-prone plants. It was later shown that karrikins act by a mechanism requiring gibberellic acid synthesis and light (Plant Physiol 2009, 149, 863). The most active karrikin is 3-methyl-2H-furo[2,3-c]pyran-2-one (see below). Due to the fact that it has an effect at extremely low concentrations (as low as 1 nM), it has potential as an important agronomic and horticultural chemical. A parent compound (3,4,5-trimethylfuran-2-one) has been also isolated from plant-derived smoke, but it has inversely a germination inhibiting property (Light ME et al., J Nat Prod 2010, 73, 267). The interaction of these compounds may have important ecological implications.
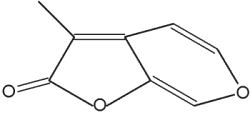
Karrikin
Brominated furanones are produced by Delisea pulchra, a marine alga endemic to the south-eastern coast of Australia. Some of which have strong inhibitory activity against fouling organisms and herbivores (de Nys R et al., Tetrahedron 1993, 49, 11213). Additionally, furanones have specific effects on colonization phenotypes of marine bacteria at concentrations found on the surface of the alga (Maximilien R et al., Aquat Microb Ecol 1998, 15, 233). Manefield M et al. have shown that these compounds inhibit acylated homoserine lactone-mediated gene expression in Escherichia coli (Manefield M et al, Microbiology 1999, 145, 283).
Quinolines, alkylquinolines and alkylquinolones
The core of quinolines is the 1-azanaphthalene nucleus. The simplest one is quinolin.
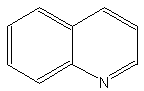
Quinolin
That compound is rarely found in living material but is present, as its derivatives, in the plant Rutales and even in some insects. Quinolin is also present in some insects, as phasmids, where it plays a role against predators.
The bacteria Pseudomonas aeruginosa was shown to produce, besides an alkyl homoserine lactone, new cell-to-cell signal molecules (quorum sensing system). This molecule was determined to have a 4-quinolone base structure with an alkyl chain (2-heptyl-3-hydroxy-4-quinolone) and therefore has been designated as the Pseudomonas quinolone signal (Pesci E et al., Proc Natl Acad Sci 1999, 96, 11229). Similar compounds have been previously described for their antimicrobial activities (Hays EE et al., J Biol Chem 1945, 149, 725). It was found that this molecule controlled the expression of genes encoding for the major virulence factors. The maximal quinolone production occurs at the end of the exponential growth phase, supporting the hypothesis that it acts as a secondary regulatory signal for a subset of quorum sensing-controlled genes.
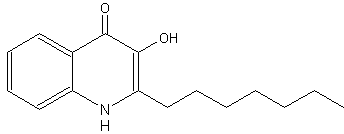
2-Heptyl-3-hydroxy-4-quinolone
Later, another analogue was isolated from the same bacteria, 3,4-dihydroxy-2-heptylquinoline, which could be the direct precursor of the previous quinolone and, likely, the message molecule involved in cell-to-cell communication (Deziel E et al., Proc Natl Acad Sci 2004, 101, 1339). A review of that type of quorum sensing may be read for further information (Dubern JF et al., Mol Biosyst 2008, 4, 882).
In contrast with Pseudomonas quinolone, Burkholderia spp produce several hydroxy-quinolines with a methyl group in position 3 and a hydroxyl group in position 4 and various alkyl chains (Vial L et al., J Bacteriol 2008, 190, 5339). It was later shown that these quorum-sensing signals control the bacterial synthesis of antibiotics (Duerkop BA et al., J Bacteriol 2009, 191, 3909).
A new quinoline-2-carboxylic acid, 4-hydroxy-6-methoxyquinoline-2-carboxylic acid (6-methoxy-kynurenic acid), has been isolated from Ephedra pachyclada. Kynurenic and 6-hydroxykynurenic acids, previously reported from plants, were also isolated from Ephedra (Starratt AN et al., Phytochemistry 1996, 42, 1477).
Compounds related to quinolines, the benzoxazinones, are present as inactive glucosides (phytoanticipines), mainly in Gramineae (rye, wheat, corn) (Niemeyer HM, Phytochemistry 1988, 27, 3349). They are sometimes described as cyclic hydroxamic acids. In rye, the principal compound is the glucoside of DIBOA (2,4-dihydroxy-1,4-benzoxazin-3-one), in wheat and corn, it is the glucoside of the methoxylated form, DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one).
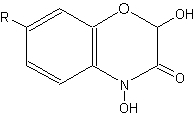
Benzoxazinones
(R = OCH3 : DIMBOA , R = H : DIBOA)
These glucosides are hydrolyzed during the plant infection by fungi or bacteria or after insect attacks. The benzoxazinones aglycones, and their breakdown products (phenoxazinones, acetamides, and malonamic acids), behave like antifungal, antibacterial and also insecticidal substances (allelopathy). These aspects of allelopathy have been reviewed for the sustainable weed control in rye fields (Schulz M et al., J Chem Ecol 2013, 39, 154).
Investigations in genetic engineering are performed to induce the production of these protective agents in other crop plants.
The nature of these compounds was discovered during the 19th century. In 1831, Wachen roder H. proposed the term "carotene" for the hydrocarbon pigment he had cristallized from carrot roots. Berzelius J. called the more polar yellow pigments extracted from autumn leaves "xanthophylls" and Tswett M., who separated many pigments by column chromatography, called the whole group "carotenoids".
Information on their physical and chemical properties as well as spectroscopic and chromatographic data for the determination of carotenoids in biological samples has been given (Rivera Vélez SM, J Nat Prod 2016, 79, 1473).
Among this important group, the numerous compounds consist of C40 chains (tetraterpenes) with conjugated double bonds, they show strong light absorption and often are brightly colored (red, orange). They occur as pigments in bacteria, algae and higher plants. Carotenoids perform three major functions in plants : accessory pigments for light harvesting, prevention of photooxidative damage and pigmentation attracting insects.
The hydrocarbon carotenoids are known as carotenes, while oxygenated derivatives of these hydrocarbons are known as xanthophylls. To date, 700 carotenoids have been identified, of which only 50 are regularly consumed in the human diet and 24 have so far been detected in human plasma. In Western diets, the most abundant carotenoids are the three oxygenated xanthophylls lutein, zeaxanthin and b-cryptoxanthin and the three major carotenes, a-carotene b-carotene and lycopene. The human intake of carotenoids may be appreciated using databases such as that establlished for Swiss vegetables (Reif C et al., J Food Comp Anal 2013, 29, 64).
Carotenoids are important components of the light harvesting in plants, expanding the absorption spectra of photosynthesis. The major carotenoids in this context are lutein, violaxanthin and neoxanthin. Additionally, there is considerable evidence which indicates a photoprotective role of xanthophylls preventing damage by dissipating excess light. In mammals, carotenoids exhibit immunomodulatory actions, likely related to their anticarcinogenic effects. b–
Carotene was thus shown to enhance cell-mediated immune responses (Hughes DA, Proc Nutr Soc 1999, 58, 713). The decrease in prostate cancer risk has been linked to the consumption of tomatoes, vegetable rich in lycopene, as prostatic tissues. While there is yet limited direct evidence linking lycopene and prostate cancer (Kavanagh CJ et al., J Natl Cancer Inst 2007, 99, 1074), several observations, including the ability of the prostate to concentrate lycopene, suggest a special protection of lycopene against that pathology (Stanley JC, Lipid technol 2008, 20, 64).
Carotenoids consist of eight isoprenoid units joined in such a manner that the arrangement of isoprenoid units is reversed at the center of the molecule so that the two central methyl groups are in a 1,6-position relationship and the remaining non-terminal methyl groups are in a 1,5-position relationship. They are, by far the predominant class of tetraterpenes. They may be also classified in the terpenoids.
Carotenoids can be considered derivatives of lycopene, found in tomatoes, fruits and flowers. Its long straight chain is highly unsaturated and composed of two identical units joined by a double bond between carbon 15 and 15′. Each of these 20 carbon units may be considered to be derived from 4 isoprene units. Lycopene is a bioactive red colored pigment naturally occurring in plants. Interest in lycopene is increasing due to increasing evidence proving its antioxidant activities and its preventive properties toward numerous diseases. In vitro, in vivo and ex vivo studies have demonstrated that lycopene-rich foods are inversely associated to diseases such as cancers, cardiovascular diseases, diabetes, and others. A review of all these aspects may be consulted (Kong KW et al., Molecules 2010, 15, 959).
Carotenoids may be acyclic (seco-carotenoids) or cyclic (mono- or bi-, alicyclic or aryl). Oxyfunctionalization of various carotenoids leads to a large number of xanthophylls in which the function may be a hydroxyl, methoxyl, carbonyl, oxo, formyl or epoxy group.
Only some of the most common carotenes and xanthophylls are given below:
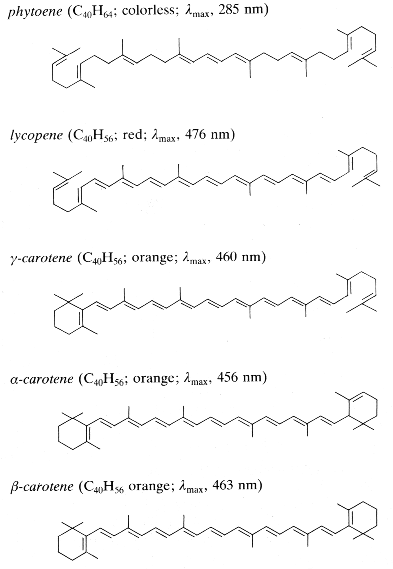
Phytoene is one of the first intermediates in the biosynthesis of carotenoids. It is formed by coupling of two molecules of geranylgeranyl pyrophosphate by the action of the phytoene synthase. The diphosphate is removed and proton shift leads to the formation of phytoene. It is a colorless product and absorbs light in the UV range only. Dietary phytoene is accumulated in human skin where it can potentially protect the skin (UV absorber, antioxidant, anti inflammatory) (Aust O et al., Int J vitam Nutr Res 2005, 75, 54).
Among the represented molecules, b-carotene is probably the most important as a precursor of vitamin A by central cleavage into retinol and some retinoic acid (b-carotene provides about 40% of human dietary retinol equivalents). The majority of carotenoids are still manufactured chemically, but, currently, the microbial production of b-carotene is of commercial importance. Blakeslea trispora is one of the best fungal strain employed for the production of b-carotene, commercially utilized by DSM. B. trispora produces up to 3% carotene per cell dry weight. B. trispora is also employed for the production of natural lycopene, commercialized by Vitatene. The marine algae Dunaliella salina is also employed for manufacturing of b-carotene (Cognis, Proalgen Biotech Ltd.).
Carotene and lycopene are known to improve skin properties when ingested as supplements or in vegetal products.They protect against sunburn, increasing the basal defense against UV light-mediated damage to the skin, although their efficacy is not comparable to the use of a sunscreen (Aust O et al., Int. J Vitam Nutr Res 2005, 75, 5460).
In human serum several carotenes and xanthophylls have been detected. If a– , b-carotene and lycopene are frequently quoted in specialized papers, some others are now determined with precise HPLC methods (lutein, zeaxanthin, cantaxanthin and b-cryptoxanthin). These compounds originate from ingested fruit, green leaves, berries and yellow corn. Egg yolk usually contains about 175-400 mg of lutein and about 200-300 g of zeaxanthin, but this could be affected by hen’s feed composition (Handelman GJ et al., Am J Clin Nutr 1999, 70, 247). Up to 18% losses of xanthophylls have been observed after various cooking methods (Nimalaratne C et al., J Agric Food Chem 2012, 60, 12547).
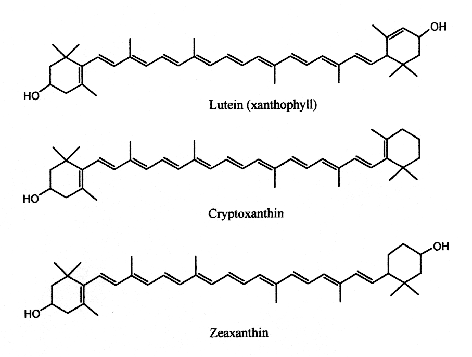
Xanthophylls
Lutein has its maximum absorption at 450 nm, cryptoxanthin at 453 nm and zeaxanthin at 454 nm.
Lutein and zeaxanthin are the only xanthophylls found in human serum that are present in retina, macula (the central region of the eye (they are frequently referred to as macular pigment), and lenses. Thus, they are at the origin of the name of the central part of the retina, macula lutea (yellow spot). By absorbing blue-light, the macular pigments protect the underlying photoreceptor cell layer from light damage, possibly initiated by the formation of reactive oxygen species. Increasing the intake of lutein or zeaxanthin may prove to be protective against the development of age-related macular degeneration (Krinsky NI et al., Annu Rev Nutr 2003, 23, 171).
Several nutritional investigations have suggested that lutein supplements may improve visual function and optical density in aged people (Olmedilla B et al., Nutrition 2003, 19, 21).
Natural aromatic derivative of lutein, 3,3′-dihydroxyisorenieratene and its non-hydroxylated parent compound, isorenieratene, are produced by green sulfur bacteria and by Brevibacterium linens, a bacteria
used in the dairy industry for production of smear cheese (Saint Paulin, Münster, St Nectaire) (Rattray FP et al., J Dairy Sci 1999, 82, 891). Their high antioxidant activity against free radicals and their specific ability to prevent oxidatively generated damage to DNA make them interesting compounds for the development of endogenous photoprotective systems (Wagener S et al., Free Rad Biol Med 2012, 53, 457).
3,3′-Dihydroxyisorenieratene (R1, R2 = OH)
Isorenieratene (R1, R2 = H)
Astaxanthin, present in yeast, microalgae, crustaceans and fish, is a natural nutritional component, used as a food supplement for human and animal consumption. Its commercial production comes from both natural and synthetic sources.
Astaxanthine
In vitro, astaxanthin is several fold more active as a free radical antioxidant than b
-carotene and a-tocopherol. In animal models, its modulates immune response (Bennedsen M et al., Immunol Lett 1999, 70, 185), inhibits cancer cell growth (Chew BP et al., Anticancer Res 1999, 19, 1849), and reduces bacterial load and gastric inflammation in vitro and rodent models. In humans, dietary astaxanthin decreases a DNA damage biomarker and acute phase protein, and enhances immune response (Park JS et al., Nutr Metabol 2010, 7, 18).
An important review has discussed the place of carotenoids in different market sectors, the methods for commercial production, the evidence supporting the health claims made by different industry sector for the most commercially valuable carotenoids on the market: b-carotene, lycopene, lutein, zeaxanthin and astaxanthin (Berman J. et al., Phytochem Rev 2015, 14, 727).
Ketocarotenoids belong to the xanthophyll group and are quite unique in nature. Among these compounds, echinenone and canthaxantin are abundant in cyanobacteria are characteristic of cyanobacteria (Takaichi S et al., Cell Mol Life Sci 2007, 64, 2607).
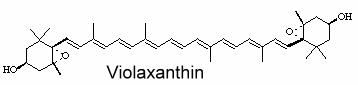
Violaxanthin is an epoxidized derivative of antheraxanthin (hydroxylated cryptoxanthin) and forms with zeaxanthin the xanthophyll cycle that is said to protect the photosynthetic system of plants against damage by excess light.
Two cycloaddition products of trans-violaxanthin with a-tocopherol have been isolated from seeds of Pittosporum tobira and their structures elucidated (Fujiwara Y et al., Tetrahedron Lett 2001, 42, 2693). These C69 carotenoids were named pittosporumxanthins. Their global structure is given below.
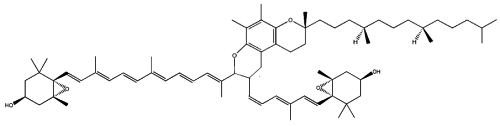
Pittosporumxanthin
Further investigations have revealed the existence of six other forms based on addition of a-tocopherol with antheraxanthin, neoxanthin or violaxanthin (Maoka T et al., J Nat Prod 2008, 71, 622).
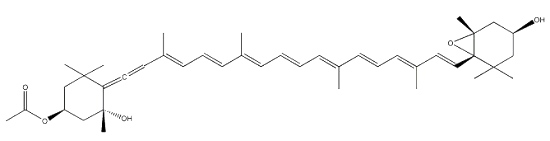
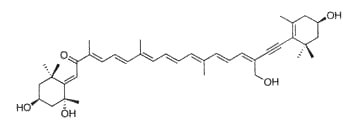
Fucoxanthin, as an allenic carotenoid with a 5,6-monoepoxide group, is one of the most abundant carotenoid in brown algae and diatoms. This compound contributes more than 10% of the estimated total production of carotenoids in nature. Fucoxanthin was first isolated by Willstätter R et al. (Ann 1914, 404, 237).
Fucoxanthin
This carotenoid has shown interesting biological properties, such as anticarcinogenetic effects, anti-inflammatory effects and radical scavenging activity. Furthermore, it has been shown that fucoxanthin has an anti-obesity effect in connection with the expression of the uncoupling protein UCP1 in white adipose tissue (Maeda H et al., Biochem Biophys Res Comm 2005, 332, 392; Miyashita K, Lipid Technol 2009, 21, 186). Its ability to enhance the docosahexaenoic acid concentration in the liver of treated obese mice remains to be examined more thoroughly (Tsukui T et al., J Agric Food Chem 2007, 55, 5025). A great part of the biological properties of fucoxanthin may be related to its ability to take up peroxynitrite through the formation of nitrofucoxanthin and the inhibition of the nitration of tyrosine by peroxynitrite (Tsuboi M et al., J Agric Food Chem 2011, 59, 10572). Therefore, fucoxanthin may have the potential to reduce the risk of cancer induced by reactive nitrogen species.
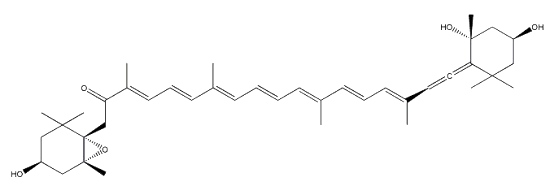
Neoxanthin is another common allenic carotenoid, widely distributed in higher plants and algae, which was first isolated from the green leaves of barley in 1938 by Strain HH.
Neoxanthin
Neoxanthin is thought to be a part of the light harvesting complexes in thylakoids and as a precursor to the plant growth hormone abscisic acid.
More than 40 allenic carotenoids have been described in vegetals and accumulated in animals (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
An unusual carotenoid ester was identified in fresh mango lipids as violaxanthin dibutyrate (Pott I et al., Phytochemistry 2003, 64, 825).
Another unusual acetylenic carotenoid was discovered and identified in a marine sponge (Prianos osiros) (Rogers EW et al., J Nat Prod 2005, 68, 450).
That new molecule was shown to be strongly cytotoxic toward cultured human colon tumor cells.
Specific carotenoids are found in mineral sediments or crude oils and are used as biomarkers. Among them, isorenieratane, found in oils of Devonian age, is derived from the carotenoid isorenieratene (an homologue of the previous one but with an unsaturated isoprenoid chain), which is synthesized by photosynthetic green sulfur bacteria (Chlorobiaceae)(Koopmans MP et al., Geochim Cosmochim Acta 1996, 60, 4467).
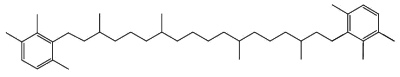
Isorenieratane
Another C40 carotenoid, paleorenieratane, also thought to be derived from the same bacteria has been identified in Devonian aged sediments and crude oils (Hartgers WA et al., Organic Geochem 1994, 22, 703).
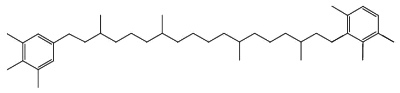
Paleorenieratane
These green sulfur bacteria are strict anaerobes that require light and hydrogen sulfide in stratified water columns to carry out photosynthesis and are thus markers for these photic zones (euxinic) in depositional environments.
C50-carotenoids, including bacterioruberin which is present in halophilic Archaea, characterize the colored saltern ponds (Lutnaes BF et al., J Nat prod 2002, 65, 1340).
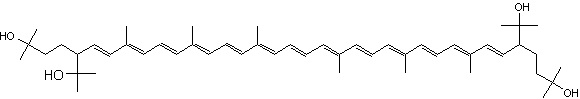
Bacterioruberin
Although green leaves contain unesterified hydroxy carotenoids, most carotenoids in ripe fruit are esterified with fatty acids. However, those of a few fruits, particularly those that remain green when ripe, such as kiwi, undergo limited or no esterification. Carotenoid mono- and diesters were identified in mandarin essential oil (Giuffrida D et al. Flavour Fragr J 2006, 21, 319), in Haematococcus pluvialis (Breithaupt DE et al., J Agric Food Chem 2004, 52, 3870)
and in the Antarctic krill Euphausia superba (Takaichi S et al. Comp Biochem Physiol B 2003, 136, 317).
Carotenoids fatty acid esters were also described in the carapace of the spiny lobster Panulirus japonicus (Maoka T et al., J Oleo Sci 2008, 57, 145). Astaxanthin, adonixanthin, and pectenolone were esterified with fatty acids with a large range of chain length and unsaturation.
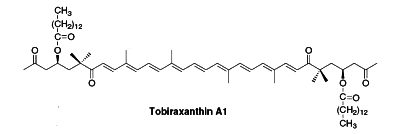
Several esterified seco-carotenoids, the tobiraxanthins, have been isolated from the seeds of Pittosporum tobira (Fujiwara Y et al., Tetrahedron Lett 2002, 43, 4385). Two fatty acid molecules (lauric or myristic acid) acylated the carotenoid part. One of these compounds is shown below (Tobiraxanthin A1).
Apocarotenoids are carotenoid derivatives formed by the removal of fragments of the carbon backbone from either or both ends of a C40 precursor such as lycopene or beta,beta-carotene. These modification originate in the oxidative degradation at the level of the terminal rings. They may be the result of nonspecific mechanisms (lipoxygenase, photo-oxidation) as well as of specific mechanisms (dioxygenases). They have significant roles as developmental and environmental response signals. They also make important contributions to flavor and nutritional quality of foods (fruits, tea, wine) and tobacco.
Two well-known apocarotenoids are bixin and crocetin which have economic importance as pigments and aroma in foods.
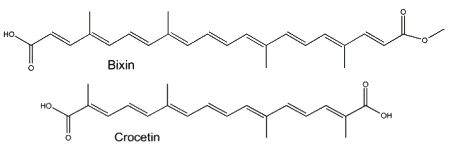
Bixin (cis- or trans-bixin) is one of several highly colored molecules extracted from annatto seed coats (Bixa orellana) and used as a food coloring and cosmetic compound since pre-Colombian times. It was determined that lycopene is the precursor of bixin (Bouvier F et al., Science 2003, 300, 2089). A review of the molecular characterization of bixin may be consulted (Ramamoorthy S et al., Ind Crops Prod 2010, 32, 48). Crocetin, occurring as various glycosyl esters, is the coloring principle of saffron, pollen harvested from Crocus sativus. This apocarotenoid originates in the rupture of zeaxanthin (Bouvier F et al., Plant Cell 2003, 15, 47). Two other C26 and C30 apocarotenoids have been characterized in the seeds of Ditaxis heterantha (a native Euphorbiaceae of Mexico), heteranthin and ditaxin (Mendez-Robles MD et al., J Nat Prod 2006, 69, 1140). The presence of these products explains the use of the Ditaxis seeds as a natural food flavor and coloring.
Carotenoids are in constant turnover involving continuous biosynthesis and catabolism, and are substrates for the
carotene cleavage dioxygenases that catalyze the cleavage of carotenoids to apocarotenoids. These enzymes cleave a broad range of carotenoids, such as lycopene, ß-carotene, d-carotene, zeaxanthin, violaxanthin, and neoxanthin, to generate aldehydes and ketones that are volatile aroma compounds (Auldridge M.E. et al., Curr Opin Plant Biol 9, 315).
These secondary metabolites are of special interest because of their possible biological functions including herbivore feeding deterrence.
It was shown that several volatile products are present after enzymatic degradation. Among them, the so-called norisoprenoids, the C13-cleavage products of carotenoids, have extremely low detection threshold values (some ng per liter of water for ionone and damascone).
Various bioassays demonstrated that ß-ionone has a strong repellent effect toward both the flea beetle and the spider mite, and significant oviposition deterrence to whiteflies. In contrast, dihydro-ß-ionone had attractant properties, especially to the crucifer flea beetle, while a-ionone did not show any significant activity (Cáceres L.A. et al., J Chem Ecol 42, 107). It may be expected that modified plants producing natural compounds with repellent or attractive effects will be exploited to reduce reliance on synthetic pesticides.
There is an enormous interest in apocarotenoids in detergent, food and perfume industry (Carotenoid-derived aroma compounds. Winterhalter P and Rouseff RL Eds, ACS Symp Series, v.802, 2002).
|
|
|
A peroxidase from edible fungi was shown to be a key enzyme able to degrade carotenoids into important flavor compounds (ionone, cyclocitral, terpineol….) (Zorn H et al., Biol Chem 2003, 384, 1049). A review of the oxidative remodeling of plastid carotenoids initiated by specific dioxygenases has been released by Camara B et al (Arch Biochem Biophys 2004, 430, 16).
Furthermore, apocarotenoids act as visual or volatile signals to attract pollinating agents, and are also key players in plant defense. Studies have shown that the loss of cleavage enzymes induces the development of axillary branches, indicating that apocarotenoids convey signals that regulate plant architecture (Bouvier F et al., Tr Plant Sci 2005, 10, 187). It appears progressively that, even in man, apocarotenoids may exert biological activities. While the mechanism of action of these compounds is not fully known, they have profound effects on gene expression through nuclear receptors (Eroglu A et al., J Lipid Res 2013, 54, 1719).
It is generally accepted that oxidation of carotenoids begins with epoxidation and cleavage to apocarotenals prior a transformation into other derivatives. Several epoxycarotenoids and apocarotenals were observed in experimental oxidation models but also some were identified in processed foods (Rodriguez EB et al., Food Chem 2007, 101, 563). The two compounds shown below were detected in fruit extracts :
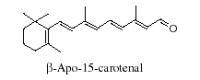

Another apocarotenal, trans–b-apo-8′-carotenal, is found in spinach and citrus fruits. It has a low pro-vitamin A activity and is used in pharmaceuticals and cosmetic products. This apocarotenal is also used as an additive (E160e) legalized by the European commission for human food.
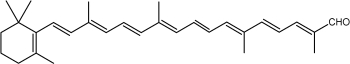
trans-b-Apo-8′-carotenal
Apolycopenals have been reported in laboratory animals consuming high lycopene diets. Apo-8′- and apo-10′-lycopenal were observed in the hepatic tissue of rats consuming a high lycopene diet (Gajic M et al., J Nutr 2006, 136, 1552). These products are not limited to mammalian systems but were also identified in extracts of tomato. Thus, apo-6′-, apo-8′-, apo-10′-, apo-12′-, and apo-14′-lycopenals were detected and quantified in tomato (6.5 mg/100 g) (Kopec RE et al., J Agric Food Chem 2010, 58, 3290), apo-12′-carotenal being the most abundant. The presence of multiple apolycopenals in the plasma of humans consuming tomato juice has been documented. This evidence suggests that these products may in fact be absorbed from the food and not solely a product of metabolism in vivo.
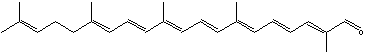
Apo-12′-carotenal
Abscisic acid is an end product of neoxanthin or violaxanthine peroxidation and reduction giving an apocarotenal with a short side chain (5 carbons), followed by a final oxidation into an acid form (Seo M et al., Trends Plant Sci 2002, 7, 41).
Trisporoids, derived from b-carotene, regulate the recognition between mating partners, early sexual morphogenesis and development in zygomycete fungi. They are released from the hyphae (sexual cells), exerting their physiological effects upon compatible mating partners. Trisporic acid and some precursors directly influence the transcription of genes involved in sexual development.
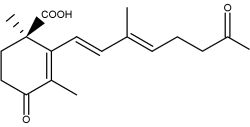
Trisporic acid
The connection between b-carotene and trisporic acid was early established when trisporic acid was established as a substance produced by mated cultures of strains of Blakeslea trispora and Mucor mucedo (van den Ende H., Nature 1967, 215, 211) and as a metabolite of b-carotene after using 14C labeled molecules (Austin DJ et al., Experientia 1970, 26, 348). As for several other b-carotene derived signal compounds, the first step in trisporoid synthesis is the oxidative cleavage of b-carotene (b-carotene oxygenases).
Strigolactones are signaling molecules that play a double role in the rhizosphere as host detection signals for arbuscular mycorrhizal fungi and root parasitic plants (Bouwmeester HJ et al., Trends Plant Sci 2007, 12, 224). In addition to their important role as rhizosphere signaling compounds, it has recently been demonstrated that strigolactones also act as new hormones inhibiting shoot branching in plants and hence are involved in the regulation of above-ground plant architecture (Gomez-Roldan V et al., Nature 2008, 455, 189; Umehara M et al., Nature 2008, 455, 195; Tsuchiya Y et al., Curr Opin Plant Biol 2009, 12, 556). Since the isolation of strigol as a germination stimulant for Striga lutea from roots of Gossypium hirsutum (Cook CE et al., J Am Chem Soc 1972, 94, 6198), more than ten strigolactones have been identified. The 5-deoxystrigol is considered as the precursor of all other strigolactones (Orobanchol, strigol).
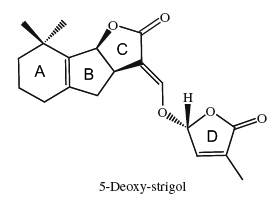
The structural core of the molecules consists of a tricyclic lactone (ABC part) that connects via an enol ether bridge to a butyrolactone group (the D-ring). All other strigolactones possess one or two methyl substituents on the A-ring and various combinations of hydroxyl or acetate substituents around the A- and B-rings. It has been demonstrated that these terpenoids are derived from the carotenoid pathway (Matusova R et al., Plant Physiol 2005, 139, 920). The biology of strigolactones has been reviewed (Ruyter-Spira C et al., Trends Plant Sci 2013, 18, 72 and Khosla A et al., Curr Op Plant Biol 2016, 33, 57). Their role in the plant defence system has been reviewed (Marzec M, Tr plant Sci 2016, 21, 900).
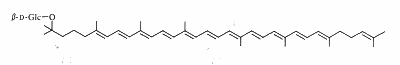
Carotenoid glycosides
Carotenoids may be linked to a sugar by a glycosidic link or an ester link. Such compounds are known for plant and bacteria. As an example, a glucoside of rhodopsin, found in the photosynthetic part of Rhodopseudomonas acidophila, is shown below.
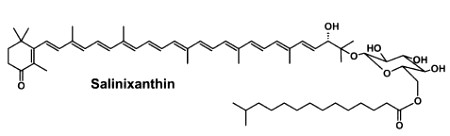
Salinixanthin is a glycosylated and acylated carotenoid associated with the protein xanthorodopsine which was isolated from the extremely halophilic eubacterium Salinibacter ruber living in salt pond in Spain (Lutnaes BF et al., J Nat Prod 2002, 65, 1340). This carotenoid has a keto group in the ring, a glycoside group and an acyl tail, probably immersed in the membrane (Balashov SP et al., Cell Mol Life Sci 2007, 64, 2323).
Astaxanthin diglucoside diesters have been determined in lipid extracts of the snow alga (Chlamydomonas nivalis) (Rezanka T et al., Phytochemistry 2008, 69, 479). The C-3 hydroxyl group of astaxanthin is glucosylated and the C-6 hydroxyl group of the glucosyl moiety is esterified with specific fatty acids. Among these fatty acids (14 species were detected), the most abundant were 16:3, 16:4, 18:1, 18:4 and 18:5.
Carotenoids are said to have antioxidant properties, as tocopherols, and thus may prevent the oxidation of the lipid moieties of LDL (low density lipoproteins) which renders these lipoproteins atherogenic.
To know more about their antioxidant properties, consult the VERIS site
The web site of the International Carotenoid Society may be consulted for further information on carotenoids.
A very informative guide to carotenoid analysis in foods has been released by Rodriguez-Amaya DB.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire