
To reduce the danger of toxicity of chloroform, Hara et al. (Anal Biochem 1978, 90, 420) described an efficient extraction procedure particularly adapted to nervous tissues.
The tissue sample is homogenized with 18 volumes of a mixture of hexane/2-propanol (3/2) for 1 minute, the suspension is filtered and the filter rinsed with 3 x 2 vol of the same solvent. As the content of non-lipids is very low (proteins, pigments, small molecules), the whole liquid phase is evaporated and the dried extract dissolved (Eder K et al. Clin Chim Acta 1993, 219, 93).
An adaptation of the Hara’s method was demonstrated to be the most efficient procedure for the extraction of plant sphingolipids (Markham JE et al., J Biol Chem 2006, 281, 22684).
Briefly, to the frozen tissue, 5 ml of the lower phase of isopropanol/hexane/water, 55/20/25 (v/v) were added. The tissue was disrupted in a glass homogenizer and transferred to a glass tube which is capped and incubated at 60°C for 15 min with occasional shaking. After centrifugation, the pellet was extracted twice more, each time with 5 ml of solvent and the supernatants were combined. A 98% recovery was obtained with leaf tissue of Arabidopsis, tomato, and soybean.
An optimized procedure for extraction of total lipids from microalgae without disruption and using a chloroform/methanol mixture has been reported (Ryckebosch E et al., JAOCS 2012, 89, 189). The recovery of lipids from microalgae by alcohol processing was determined with two species, Nannochloropsis and Schizochytrium (Wang G et al., JAOCS 2012, 89, 335).
Accurate profiling of lipidomes were obtained by MTBE extraction which allows faster and cleaner lipid recovery (Matyash V et al., J Lipid Res 2010, 49, 1137). Because of MTBE’s low density, lipid-containing organic phase forms the upper layer during phase separation, which simplifies its collection and minimizes dripping losses. Nonextractable matrix forms a dense pellet at the bottom of the extraction tube and is easily removed by centrifugation. Rigorous testing demonstrated that the MTBE protocol delivers similar or better recoveries of species of most all major lipid classes compared with the Folch or Bligh and Dyer recipes.
Briefly, methanol (1.5 ml) was added to a 200 ml sample aliquot, and the tube was vortexed. Then, 5 ml of MTBE was added and the mixture was incubated for 1 h at room temperature. Phase separation was induced by adding 1.25 ml of water. Upon 10 min of incubation at room temperature, the sample was centrifuged at 1,000 g for 10 min. The upper organic phase was collected, and the lower phase was re-extracted with 2 ml of MTBE/methanol/water (10/3/2.5, v/v/v). Combined organic phases were dried in a vacuum centrifuge. Extracted lipids were dissolved in 200 ml of CHCl3/methanol/water (60/30/4.5, v/v/v) for storage.
A similar procedure has been described before analysis of blood lipid classes (Ichihara K et al., Lipids 2011, 46, 297). This extraction has been also proposed for the analysis of human brain lipids (Abbott SK et al., Lipids 2013, 48, 307). This study leads to the conclusion that this protocol, including a mechanical homogenization utilizing ceramic beads, is equivalent to the traditional Folch protocol for lipid extraction and quantification of glycerophospholipid, sphingolipid and sterol species in human brain tissue.
Extraction of all lipids with hexane
A very precise investigation of the effects of temperature and contact time on extraction efficiency of sunflower cake was reported using hexane as solvent (Baümler ER et al., JAOCS 2010, 87, 1489). Extraction at 60°C during 30 min leads to a very high yield (99%) for triacylglycerols and tocopherols and to a reduced phospholipid extraction (66%).
Extraction of dry tissue lipids
Based on precise studies, it has been shown that freeze-dried tissues (krill) extracted with a one-step procedure (acetone:ethanol, 1:1, v:v) using 1:12 krill:solvent ratio (w:v) resulted in the highest oil extraction efficiency (Gigliotti JC et al., Food Chem 2011, 125, 1028). That oil contained about 30% of phospholipids, 3% of triacylglycerols and 67% of polar non-phospholipid classes (cholesterol, mono- and diacylglycerols, astaxanthin) .
An alternative to the traditional Folch method was described using solvent elution of a dry column composed of a tissue sample, anhydrous sodium sulfate, and Celite diatomaceous earth ground together (Marmer WN et al. Lipids 1981, 16, 365). Alternatively, lipids may be isolated and simultaneously separated into neutral and polar fractions by a sequential elution procedure. Analyses of muscle and adipose tissues demonstrated that results were similar to those obtained with chloroform/methanol methods.
A less time-consuming dry-column method was scaled down and adapted to 1g samples (liver and muscle tissues) (Elmer-Frohlich K et al., JAOCS 1992, 69, 243).
Procedure: The sample (about 1 g) is ground for 30 sec. in an ice-chilled mortar with 4 g anhydrous Na2SO4 and 0.1 ml BHT (20 mg/l dichloromethane). Celite 545 (3 g) (Fisher Scientific) is added and the mixture is ground for an additional 30 sec. to obtain a fine homogenized powder.
The powder is poured into a 16 mm X 30 cm glass column packed with glass wool and 2g of CaHPO4/Celite 545 (1/9 w/w) at its tip. A slight compression was accomplished at the top with a glass rod. Mortar, pestle, and glass rod are rinsed with 15 ml of dichloromethane/methanol (9/1, v/v) which are transferred into the column. In addition to these 15 ml, 50 ml of solvent mixture are added to elute lipids which are isolated, weighed and analyzed after evaporation of the solvent under nitrogen flushing.
Multiple columns may be run simultaneously.
A patented process, first reported in 1989 (Barker SA et al., J Chromatogr 1989, 475, 353), known as “matrix solid-phase dispersion” was described to conduct simultaneously disruption and extraction of solid and semi-solid samples. Thus, a highly viscous, semi-solid or solid sample can be placed in a mortar containing a bonded-phase solid support material (C18 bonded silica) and mechanically blended to perform a complete disruption and dispersal of the sample. This blend is sufficiently dry to transfer and pack into a column for more classical application of solid-phase extraction to the isolation of sample components. This technique has been most frequently applied to the isolation of drugs, herbicides, pesticides and other pollutants from animal tissues, fruits and vegetables (review in : Barker SA, J Chromatogr A 2000, 885, 115).
The method described by Soxhlet in 1879 is the most commonly used example of a semi-continuous method applied to extraction of lipids from foods. According to the Soxhlet’s procedure, oil and fat from solid material are extracted by repeated washing (percolation) with an organic solvent, usually hexane or petroleum ether, under reflux in a special glassware.
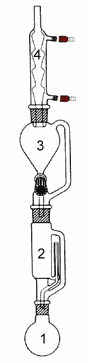
In this method the sample is dried, ground into small particles and placed in a porous cellulose thimble. The thimble is placed in an extraction chamber (2), which is suspended above a flask containing the solvent (1) and below a condenser (4). The flask is heated and the solvent evaporates and moves up into the condenser where it is converted into a liquid that trickles into the extraction chamber containing the sample. The extraction chamber is designed so that when the solvent surrounding the sample exceeds a certain level it overflows and trickles back down into the boiling flask. At the end of the extraction process, which lasts a few hours, the flask containing the solvent and lipid is removed. In some device a funnel (3) allows to recover the solvent at the end of the extraction after closing a stopcock between the funnel and the extraction chamber. The solvent in the flask (1) is then evaporated and the mass of the remaining lipid is measured. The percentage of lipid in the initial sample can then be calculated.
Despite disadvantages of this procedure (poor extraction of polar lipids, long time involved, large volumes of solvents, hazards of boiling solvents), several methods involving automatic solvent extraction were described. Different automated or semi-automated extraction instruments may be found on the market.
Several solvent extraction systems based on the Soxhlet device are on the market to allow fast and safe determination of total lipids in food, soil, ….
As an example, FOSS has launched several types of “Soxtec Systems” including automated or semi-automated analyzers, which extract lipids rapidly and accurately.
These instruments perform boiling, rinsing and solvent recovery. Similarly, Soxtherm extractors from Gerhardt GmbH was developed to reduce extraction times.
The sample to be analyzed is weighed into cellulose thimbles and inserted in the extraction device. Except diethyl ether, all solvents may be used (about 15 ml per sample), with a 75% recovery of the solvent after the extraction which is completed in 30 to 60 min, depending on the application.
Another device by ViscoALPHA enables the user to have a 2, 4 or 6 place system in 2 versions (micro or macro). An electronic unit can control and monitor up to 4 extraction units individually.
Compact and simple systems with one to six samples are sold by Behr Labor-Technic GmbH.
The XT10 Extractor by ANKOM provides an Official Procedure (AOCS Am 5-04) for efficient and economical solvent extractions of various sample types. It allows batch processing for the extraction of up to fifteen samples at a time and up to 100 samples per day. A process temperature of 90°C accelerates extraction kinetics, reducing most extraction times to under 40 minutes. Samples are placed into filter bags which prevent sample transfer error. The XT10 automatically recycles solvent and makes it ready for reuse. Solvent is manually added after each extraction. The XT15 Extractor provides a totally automatic operation for up to 150 samples.
The Büchi Extraction System B-811 is an automated system which can be used to perform an extraction according to the original Soxhlet principle. Four different extraction methods are possible without making any changes to the unit : Soxhlet standard, Soxhlet warm, hot extraction and continuous extraction. The system has an inert gas supply to avoid oxidation during extraction and to accelerate the evaporation and drying process even with high boiling point solvents (up to 150°C). Several application notes may be downloaded from the Büchi site.
A comparison of different extraction methods for total lipid quantification in meat and meat products was reported (Perez-Palacios T et al., Food Chem 2008, 110, 1025). The Soxhlet method with previous acid hydrolysis had the same efficiency as that of the method described by Folch.
A microwave-assisted Soxhlet extraction of seed oil (sunflower, soybean, rape) was described using a cellulose cartridge placed into a quartz extraction vessel inserted in a modified Microdigest 301 device (Prolabo). This procedure is slightly different from that described for the extraction of dry materials. Despite a 3 h analysis, the time reduction and the lack of need for seed grinding makes this procedure a suitable competitor of the previously described methods (Garcia-Ayuso LE et al., Anal Chem 1998, 70, 2626; Garcia-Ayuso LE et al., Chromatographia 2000, 52, 103). Comparative experiments have shown that no significant differences between the extract obtained by the Folch reference method without fat alterations and the microwave-assisted Soxhlet extraction were detected. These results demonstrated the applicability of that method for the extraction step in routine analysis of a great quantity of food samples (Ruiz-Jimenez J et al., Anal Chim Acta 2004, 525, 159).
This extraction technique, called microwave-assisted Soxhlet extraction, uses two sources of energy, namely microwaves, applied on the extraction chamber of a modified Soxhlet, and electrical heating applied on the distillation flask. This system has been used for the determination of oil content and fatty acid composition of various biological materials and foodstuffs. To overcome some limitations of the analytical process (water content), a new and convenient process was designed and developed (Virot M et al., J Chromatogr A 2007, 1174, 138; Virot M et al., J Chromatogr A 2008, 1196-1197, 57).
A modification of that extraction procedure was proposed taking into account the use of a “green solvent”, limonene, instead of hexane (Virot M et al., J Chromatogr A 2008, 1196-1197, 147). The proposed method is effective and valuable since no significant difference was obtained when using hexane or limonene for the extraction of oleaginous seeds.
A critical evaluation of various solvents systems used for the quantification of microalgal fatty acids with lyophilized samples has been reported (McNichol J et al., Lipids 2012, 47, 195).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire