
There are essentially two types of phenolic lipid, those in which the side-chain is isoprenoid, as in α-tocopherol, and those which are non-isoprenoid (“polyketide origin”) but with a normal carbon chain. The former are described in another site part, the later are described below.
The simplest form of phenolic lipids may be that of benzoic acid, the simplest aromatic carboxylic acid. The name is derived from gum benzoin (balsamic resin obtained from the bark of several species of trees in the genus Styrax) which was for a long time its only source. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. Its composition has been determined by the great German chemists Justus von Liebig and Friedrich Wöhler.
Benzoic acid is widely used as a preservative in the food, pharmaceutical, and cosmetics industries. It has very powerful antimicrobial and antioxidant properties. In the food industries, it is widely used as preservative at pH less than 4.5 due to its low cost and easy incorporation into products. In medicine, it was used as an expectorant, analgesic, and antiseptic in the early 20th century.
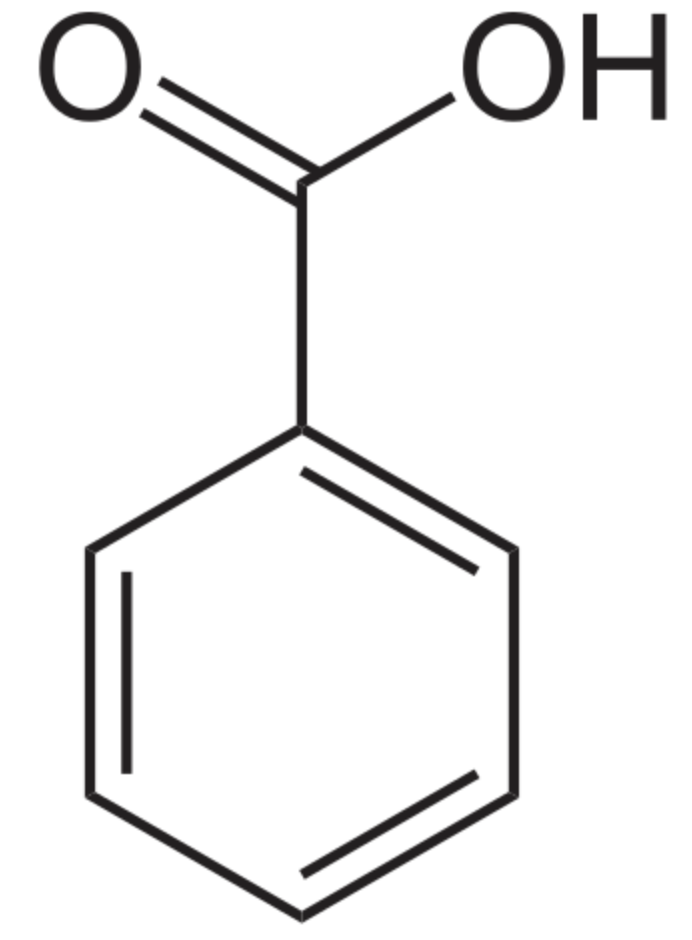
Benzoic acid
Butylated hydroxytoluene, also known as dibutylhydroxytoluene, is a derivative of phenol, that is useful for its antioxidant properties. In several countries, regulations allow small amounts to be used as a food additive. It is permitted in the European Union under E321. Industrially, While that antioxidant is chemically prepared by the reaction of p-cresol with isobutylene catalyzed by sulfuric acid, it has been found in phytoplankton, including green algae and cyanobacteria. Besides other phenolics, butylated hydroxytoluene was detected as a natural antioxidant in litchi pericarp (Jiang G et al., Foof Chem 2013, 136, 563). It behaves as a synthetic analog of vitamin E, primarily acting as a terminating agent that suppresses autoxidation by converting peroxy radicals to hydroperoxides.
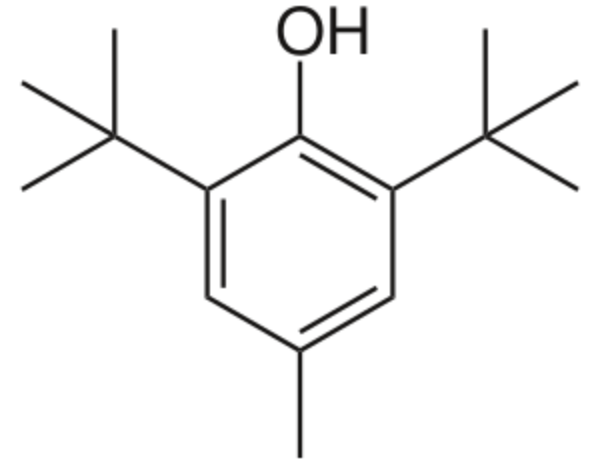
Butylated hydroxytoluene
Butylated hydroxyanisole is an antioxidant consisting of a mixture of two isomeric organic compounds, 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. It is chemically prepared from 4-methoxyphenol and isobutylene. As a food additive it has the E number E320. The primary use for butylated hydroxyanisole is as an antioxidant and preservative in food, cosmetics, medicines, rubber, and several petroleum products. The U.S. National Institutes of Health report that it could be a human carcinogen based on evidence of carcinogenicity in experimental animals.
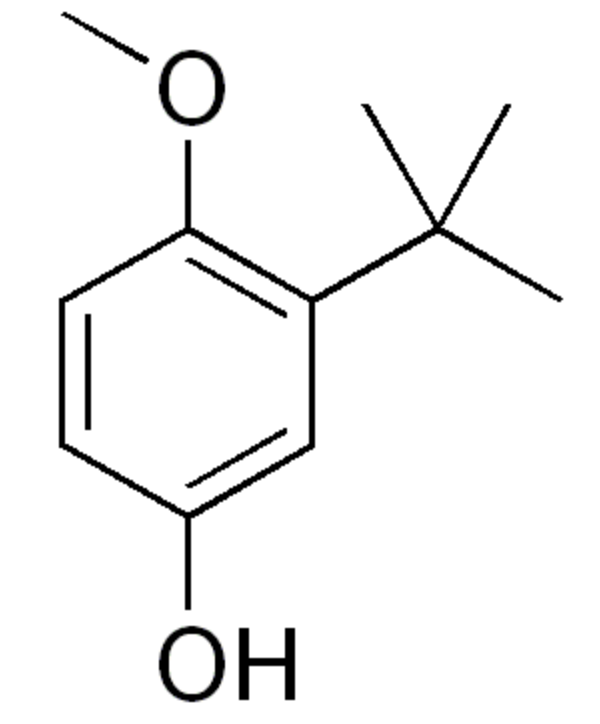
2-Butylated hydroxyanisole
Most complex phenolic compounds are mainly present in plants. This heterogeneous group includes simple phenols and polyphenols as well as their derivatives and may be classified into coumarins, quinones, and flavonoids, by far the largest group of phenolics. Among single-ring compounds those considered as lipids are found specifically in some plants and contain a catechol, a resorcinol or a hydroquinone nucleus alkylated by a normal carbon chain.
They are in general toxic and present mainly in one important plant family, the Anacardiaceae, causing serious allergic dermatitis. This family includes the well known genera: Toxicodendron (poison ivy, poison sumac, Japanese lacquer tree), Anacardium (cashew), and Mangifera (mango). Similar allergenic lipids are also found in Proteaceae (Ginkgo).
Phenylpropanoids
Among short-chain alkylphenols, the phenylpropanoids are components found in essential oils. They constitute a group of organic compounds synthesized by plants via the shikimate pathway; the aromatic amino acid L-phenylalanine is their biogenetic precursor. The core of phenylpropanoids consists of a phenyl ring connected to a C3 propane moiety. They are found in the plant kingdom, but are less common than terpenes. Several were identified in Daucus crinitus (Apiaceae). Thus, isochavicol and derivatives (butyrate, isopropionate and 2-methylbutyrate) have been characterized (Lanfranchi DA et al., J Agric Food Chem 2010, 58, 2174). Isochavicol displayed an interesting antiplasmodial activity.
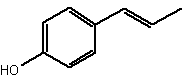
Isochavicol
Eugenol is a member of the allylbenzene class of chemical compounds (or phenypropene). This compound is extracted from some essential oils especially from clove (species Eugenia, in concentrations of 80–90%), nutmeg, cinnamon, basil and bay leaf. It is known to possess anesthetic properties. anti-oxidant and anti-inflammatory activities but at higher concentrations it acts as an oxidant and potent allergen. It was shown that bis-eugenol synthesized by the oxidation of eugenol was less cytotoxic and more highly antioxidative than eugenol (Murakami Y et al., Biochem Pharmacol 2003, 66, 1061). A review focused on anticancer potential and other pharmacological aspects of eugenol through possible mechanisms (Noman AM et al., Food Sci Nutr 2025, 13, e70727).
Like many other anesthetic agents, this 2-alkyl(oxy)phenol acts as positive allosteric modulator of the GABAA receptor. Eugenol is used as a flavor or aroma ingredient and also as a local antiseptic and anaesthetic (dentistry), pesticide and fungicide. It is attractive to males of various species of orchid bees which metabolized it into pheromones. Eugenol has in vitro an antileishmanial activity against Leishmania mexicana promastigotes with an IC50 of 2.72 µg/mL and a high selectivity index. Leishmaniasis is a neglected tropical disease that still infects thousands of people per year throughout the world (Islamuddin M et al., PLoS Negl. Trop. Dis. 2016, 10, e0005011). Research have suggested a practical use of basil as companion plants in organic crop production (Rahimian S et al., Agric Envir Chem July 4, 2025). Thus, cocultivation of common bean (Phaseolus vulgaris) with bush basil (Ocimum basilicum) enhanced the defense capacity of P. vulgaris against spider mites (Tetranychus urticae) and field herbivores. Eugenol was likely responsible for conferring the antiherbivore activity on the receiver plants.
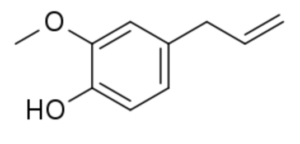 Eugenol
Eugenol
Numerous phenylpropanoids are derivatives of cinnamic acid. Their structure is based on the type C6-C3. The hydroxyl groups of derivatives (hydroxycinnamic acids) are frequently methylated (-O-CH3). Cinnamic acid is obtained from oil of cinnamon (ethyl cinnamate), some balsams (styrax from Liquidambar) and shea butter. It is used as a precursor for several derivatives for the perfume industry.
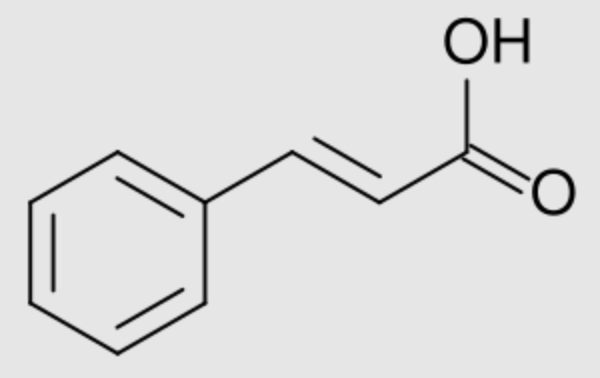 Cinnamic acid
Cinnamic acid
Sinapinic acid (or sinapic acid) is a small naturally occurring hydroxycinnamic acid.
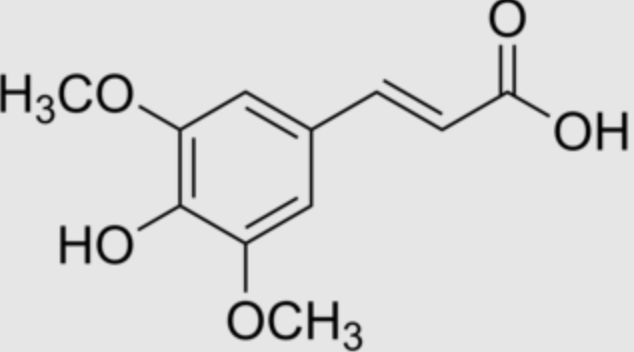 Sinapic acid
Sinapic acid
Canolol is a phenolic compound found in crude canola oil and produced by decarboxylation of sinapic acid during canola seed roasting. It has been shown to possess strong antioxidant activity, the ability to neutralize free radicals, inhibit lipid peroxidation, and down-regulate pro-inflammatory cytokines such as TNF-α and IL-6 (Ma C et al., J Agric Food Chem. 2025, 73, 9103). Additionally, it has demonstrated anticancer and neuroprotective effects (Xia X et al., Chem Biol Interact 2019, 300, 138). Canolol and its dimer from rapeseed oil was shown to attenuate advanced glycation end products-induced endothelial cytotoxicity via modulation of MAPK/NF-κB signaling axis (Liu H et al., Food Biosci 2025, 73, 107604). Thus, it offers a potential approach for preventing and managing diabetes-related cardiovascular diseases.
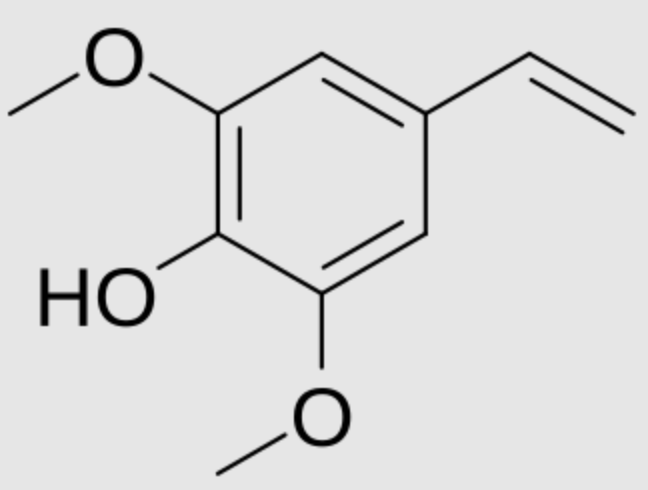 Canolol
Canolol
Sinapyl alcohol is a metabolite of sinapic acid. It is one of the three main monolignols, it is a monomeric form of lignin and lignan, both being the key structural materials in the support tissues of most plants.
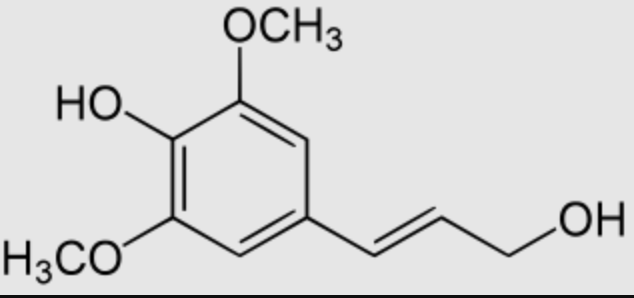 Sinapyl alcohol
Sinapyl alcohol
Sorbicillinoids are a growing class of natural products that are found in a variety of fungi including members of the orders Hypocreales and Eurotiales. The majority which are named after the common hexaketide precursor sorbicillin, exhibit anti-inflammatory, antimicrobial, cytotoxic, phytotoxic, and other selective enzyme inhibitory activities. The first representative of this class of natural products, sorbicillin, was initially isolated and characterized in 1948 by Cram from Penicillium notatum. The structures, bioactivities, biosynthesis, and synthesis of these compounds have been reviewed (Milzarek TM et al., Nat Prod Rep 2025, 42, 482).
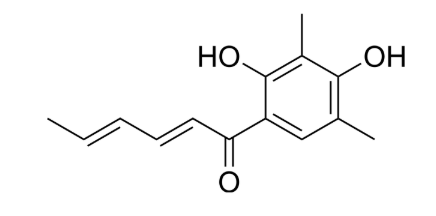 Sorbicillin
Sorbicillin
Xanthohumol is a natural product found in the female inflorescences of hops (Humulus lupulus) but also in beer. It contribute to the bitterness and flavor of hops. This prenylated chalconoid is synthesized from phenylalanine (Wikipedia) and has received much attention from researchers due to its potential cancer chemopreventive action (Gerhäuser C, Eur J Cancer 2005, 41, 1941) and its broad-spectrum antibacterial and antifungal properties (Zhang Q et al., Food Biosci 2025, 63, 105767).
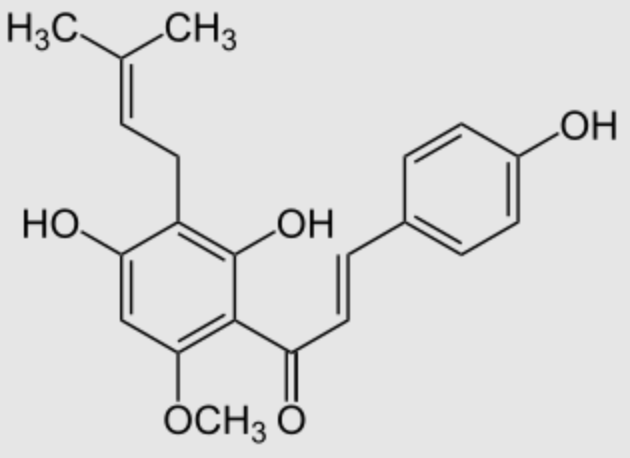 Xanthohumol
Xanthohumol
Some alkylphenols are produced by industrial synthesis to be transformed into polyethoxylate detergents (surfactants, emulsifiers) and pesticides. Because of their man-made origins, these alkylphenols are classified as xenobiotics. The most frequently used are octyl- and nonylphenols, the hydrocarbon chain of eight or nine carbon atoms is attached to the phenol ring in either the ortho, meta, or para position. Moreover, the alkyl chains may be either linear (n-alkyl chain), or branched.
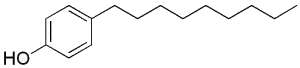
Nonylphenol
As, in animals, they mimic the effect of estrogens, they are part of the xenoestrogen group. Their potential ecological and human health impact is under study.
Bisphenol A is a chemical compound primarily used in the manufacturing of various plastics (Wikipedia). It is a colourless solid produced on an industrial scale by the condensation reaction of phenol and acetone. Its main application is as a co-monomer in the production of polycarbonates, epoxy resins and vinyl ester resins. Despite scientific debate, it appears as a xenoestrogen, exhibiting hormone-like properties that mimic the effects of estrogen in the body. That property has been detected by the British biochemist EC Dodds in the early 1930s. Some manufacturers have replaced bisphenol A by bisphenol S and bisphenol F.
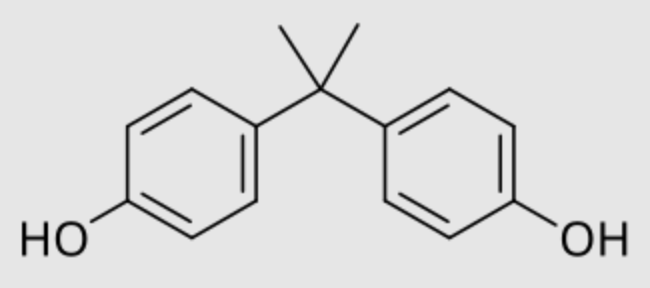 Bisphenol A
Bisphenol A
Bisphenol A diglycidyl ether is a key component of many epoxy resin formulations. It is prepared by O-alkylation of bisphenol A with epichlorohydrin. Bisphenol A diglycidyl ether-based epoxy coatings are extensively used for coating the inside of cans which come into contact with food (Wikipedia).
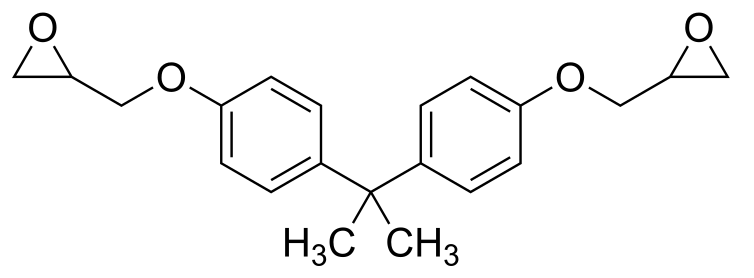 Bisphenol A diglycidyl ether
Bisphenol A diglycidyl ether
Parabens belong to a series of parahydroxybenzoates or esters of parahydroxybenzoic acid with an alkyl chain such as methyl (E218 et E219), ethyl (E214 et E215), propyl (E216 et E217) or butyl.
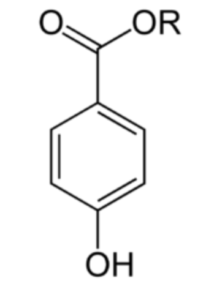 Parabens (a para-hydroxybenzoate with R = alkyl group)
Parabens (a para-hydroxybenzoate with R = alkyl group)
Although some are identical to those found in nature in very low concentrations, they are synthetically produced by the esterification of para-hydroxybenzoic acid with the appropriate alcohol. These chemicals are commonly used as preservatives in cosmetic and pharmaceutical products, their antibacterial and antimycosic mode of action being not well understood. They are also used as food preservatives. Their common inclusion in consumer products is due to their favorable lipophilic properties.
Several research programs ask questions and evaluate data about parabens’ possible health effects (Wikipedia). The use of parabens as food preservatives is regulated differently from country to country. The European Scientific Committee on Consumer Safety stated that methylparaben and ethylparaben are safe at the maximum authorized concentrations (up to 0.4% for each ester or 0.8%). It was concluded that the use of butylparaben and propylparaben as preservatives in finished cosmetic products is safe to the consumer, as long as the sum of their individual concentrations does not exceed 0.19%. Isopropylparaben, isobutylparaben, phenylparaben, benzylparaben and pentylparaben have been banned by European Commission Regulation.
A review has compiled and analyzed the available scientific evidence on the quantitative concentrations of parabens in food samples worldwide without temporal restrictions (González-Palacios P et al., Food Chem 2025, 496, 146605).
Magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl) is an organic compound that is classified as lignan and found in the bark of some species of magnolia (Magnolia officinalis or grandiflora). The extracts of these barks have been used in traditional Chinese and Japanese medicine. Magnolol is known to act on the GABAA receptors but has also osteoblast-stimulating and osteoclast-inhibiting activities. Magnolol is also binding in dimeric mode to PPARγ, acting as an agonist of this nuclear receptor. Many investigators reported the anti-cancer, anti-stress, anti-anxiety, anti-depressant, anti-oxidant, anti-inflammatory and hepatoprotective effects (review in Lee YJ et al., Paharmacol Therapeut 2011, 130, 157). It has been shown that honokiol and magnolol may bind to estrogen receptors ERα and ERβ. Biological testing confirmed that these compounds modulate estrogen-regulated transcriptional activity mediated by ERα or ERβ with potencies in the nanomolar range (Tinivella A et al., J Nat Prod 2024, 87, 2568). Magnolol was found to have a strong antifungal activity against Trichoderma hamatum which infects.sweet potato storage roots, demonstrating an excellent protective efficacy against that important pathogen-induced disease (Peng G et al., J Agric Food Chem 2025, may 23). In addition to magnolol, honokiol (an isomer of magolol) is present.in magnolia extracts. It is a small, hydrophobic neolignan biphenol structurally similar to propofol. As it can readily cross the blood-brain and cerebrospinal fluid barriers, it is highly bioavailable and potentially effective therapeutic agent (Wikipedia). Research have emphasized a new mechanism of honokiol-mediated radioprotection through the TrxR/Trx redox pathway and underlined the broad radiation-protective potential of natural product (Chen Y et al., Free Rad Biol Med 2025, 238, 235). A comprehensive review of anti-cancer mechanisms of polyphenol honokiol has been released (Solanki R et al., Phytochem Rev 2025, 24, 5701).
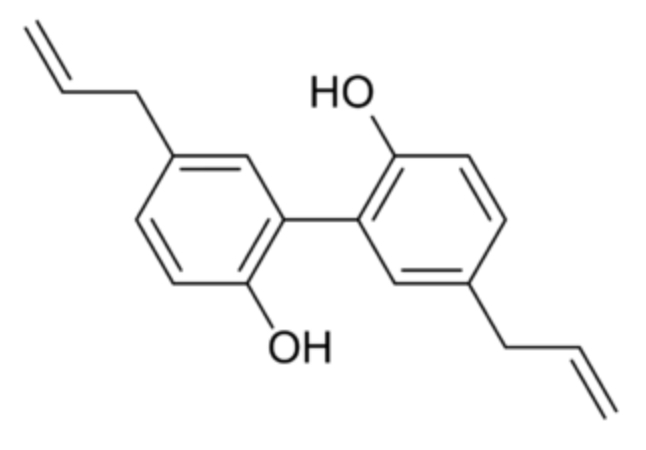
Magnolol
The molecular mechanisms, toxicity, bioavailability, and formulations of magnolol have been summarized (Lin Y et al., Front Pharmacol 2021, 17 March).
Among the polyphenol compounds there is triphenyl phosphate. This triester of phosphoric acid and phenol is present as a pollutant in animals and near all the environment since It is largely used as a plasticizer and a fire retardant. The exposure to triphenyl phosphate has been linked with reproductive and developmental toxicity, neurotoxicity, metabolic disruption, endocrine effects, and genotoxicity. The European Chemicals Agency considers this pollutant to be “very toxic” to aquatic life, with potentially long-lasting effects (Wikipedia). Worse, epidemiological studies have shown that it can accumulate in the placenta, and positively correlated with abnormal birth outcomes (Wang Y et al., Chemosphere 2021, 275, 129978).
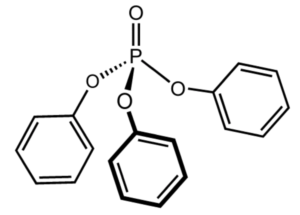 Triphenyl phosphate
Triphenyl phosphate
Vanillin, as other vanilloids, is a phenolic aldehyde. Their characteristic functional group consists of a benzylic ring substituted by a hydroxyl group (phenol) in the para position and a methoxy group in the meta position. The vanilloid group includes vanillin, vanillic acid, gingerol, shogaol, capsaicin, vanillylmandelic acid, etc.
Vanillin is the primary component of the extract of the vanilla bean. Vanillin is the most widely produced flavouring in the world, far ahead of chocolate and coffee flavourings. Vanilla flavouring production is estimated at 25,000 t/year. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals. Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin.
Vanillin was first isolated in 1858 by the French chemist Théodore Nicolas Gobley and its chemical structure was elucidated In 1874 by the German scientists Ferdinand Tiemann and Wilhelm Haarmann. At the same time, they proposed its synthesis from isoeugenol found in pine bark. Later, vanillin was synthesized from lignin-containing “brown liquor”, a byproduct of the sulfite process for making wood pulp. Today, most vanillin is produced from guaiacol and more than half the world’s vanillin production is used in the synthesis of other chemicals.
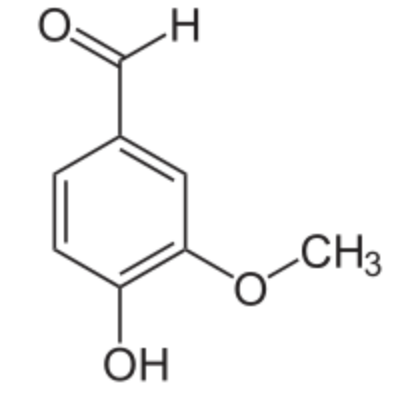 Vanillin
Vanillin
Divanillin is a dimer metabolite of vanillin appearing during its preparation under the action of oxidative enzymes such as peroxidases. Divanillin is approved for use in food as a flavor enhancer in dairy products, as it imparts a pleasant and creamy sensation to foods when added at concentrations of 5–50 mg/L In addition to being an excellent antioxidant, it has demonstrated antitumor capabilities (de Oliveira GS et al., Nat Prod Res 2024 Sep 1, 1-6).
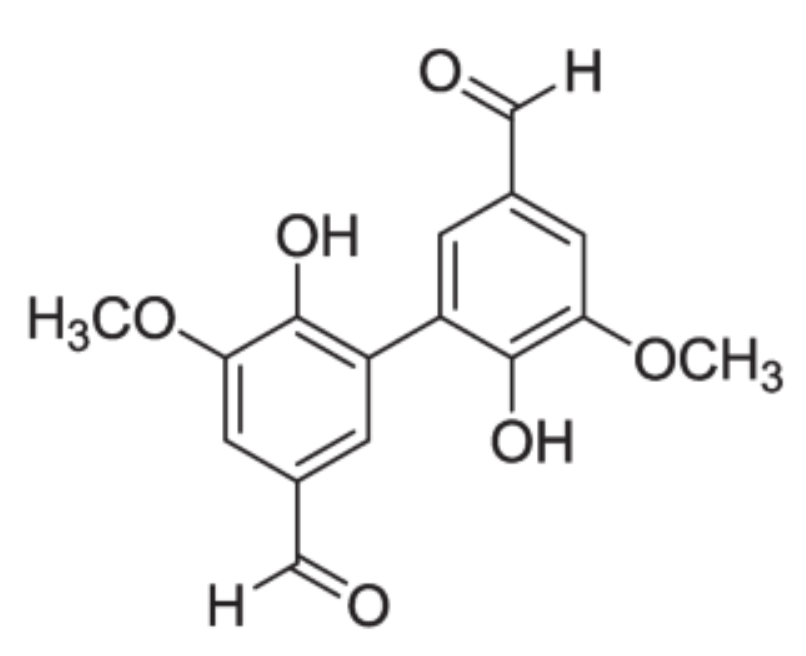 Divanillin
Divanillin
Shogaol, or [6]-shogaol, is a phenolic compound of the vanilloid family, and the main bioactive compounds of dried ginger. It has a pungent taste, as vanillin, vanillic acid, and capsaicin. The name shogaol is derived from the japonese name of ginger, plant which contains a parent compound, 6-gingerol. Gingerol has been shown to attenuates lipopolysaccharide-induced oxidation, inflammation, cognitive deficits, neuroplasticity, and amyloidogenesis in rat (Adetuyi BO et al., J Food Biochem 2021, 45/4, e13660).
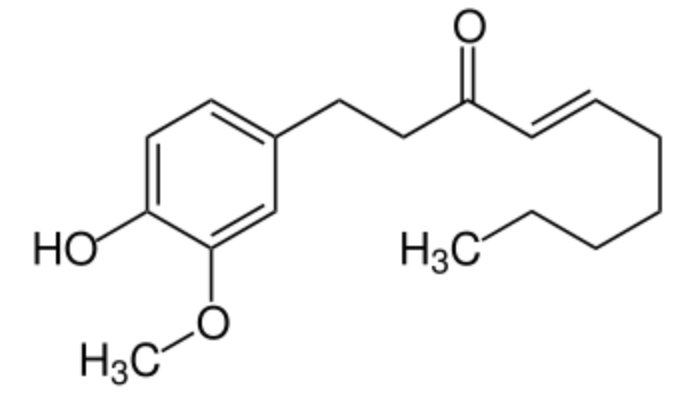 Shogaol
Shogaol
Studies have demonstrated that 6-shogaol induced neuritogenic activity in PC-12 cells via the activation MEK/ERK1/2 and PI3K/AKT signaling pathways. This suggests that 6-shogaol could act as an NGF mimic, which may be beneficial for preventive and therapeutic uses in neurodegenerative diseases (Seow S et al., BMC Complement Altern Med volume 2017, 17, 334). 6-Shogaol is a potential neuro-nutraceutical which is known to ameliorate motor symptoms by suppressing inflammation in Parkinson’s disease mice (Kim JH et al., Eur J Nutr 2025, 64, 116). Further findings obtained in animals highlighted 6-shogaol as a promising functional food component for managing Parkinson’s disease symptoms and targeting key pathological processes (Jin J et al., J Agric Food Chem 2025, November 29).
6-Gingerol and 6-shogaol have been shown to attenuate chemotherapy-induced nausea and vomiting in attenuating mitochondrial dysfunction (Zhai Y et al., Food Biosci 2025, 69, 106808).
6-Shogaol, was also shown to inhibit the growth of a variety of human cancer cells and especially cervical cancer cells (Pei XD et al., Eur J Nutr 2021, 60, 2781).
Biological mechanisms, pharmacological and pathological activities, and quality optimization of gingerols and shogaols have been reviewed (Jiang X, J Funct Foods 2025, 127, 106773).
Zingerone, also called vanillylacetone, is a major flavor component of ginger, providing the sweet flavor of cooked ginger. The chemical structure of zingerone is similar to that of other flavor chemicals such as vanillin and eugenol. It is produced by cooking or drying of the ginger root, Zingerone was shown to be a particularly efficient free radical scavenger. It is produced by some orchid flowers to attract pollinating fruit flies by mimicking the sex pheromone of the fly (Keng-Hong T et al., Biochem Syst Ecol 2007, 334).
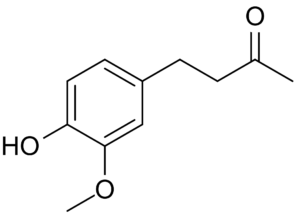 Zingerone
Zingerone
Among phenylpropanoids, chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. They have a common chemical scaffold of 1,3-diaryl-2-propen-1-one, that exists as trans and cis isomers. Plant-derived compounds, they are intermediates in the synthesis of many biological compounds. Thus, the closure of hydroxychalcones causes the formation of the flavonoid structure. Chalcone is also a member of styrenes family.
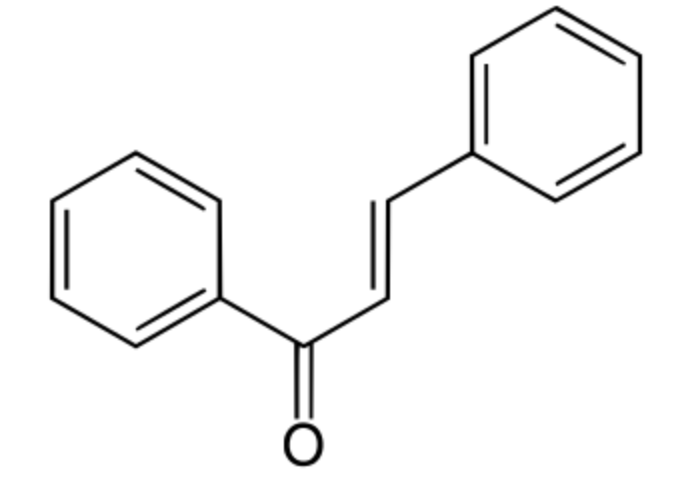 Chalcone
Chalcone
The chalcone family has attracted much interest due to its broad interesting biological properties (antibacterial, antifungal, antitumor and anti-inflammatory). Chalcone derivatives of diverse chemical architectures are quite significant in anticancer drug discovery and hence are in the center of attention of drug hunters. More than 92,000 chalcones can be found data bases and over 1,000 of them have biological data. Since many years, they were extracted from plants and herbs and used for the treatment of different medical disorders, such as cancer, inflammation, and diabetes (Chunlin Z et al., Chem Rev 2017, 117, 7762). Several chalcone-based compounds have been approved for clinical use.
Cinnacassdiol has been isolated from the barks of Cinnamomum cassia (cinnamon, or Chinese cinnamon) a classic plant with both medicinal and culinary value and highly recognized in the traditional Chinese medicine for its significant anti-inflammatory properties. It was described as a phenylpropanoid-glycosyl derivative and structurally characterized (Feng H et al., J Funct Foods 2025, 133, 107001). This phenylpropanoid exhibited distinct anti-inflammatory activities inhibiting the expressions of inflammatory cytokines. It could be a promising candidate to serve as a functional food ingredient.
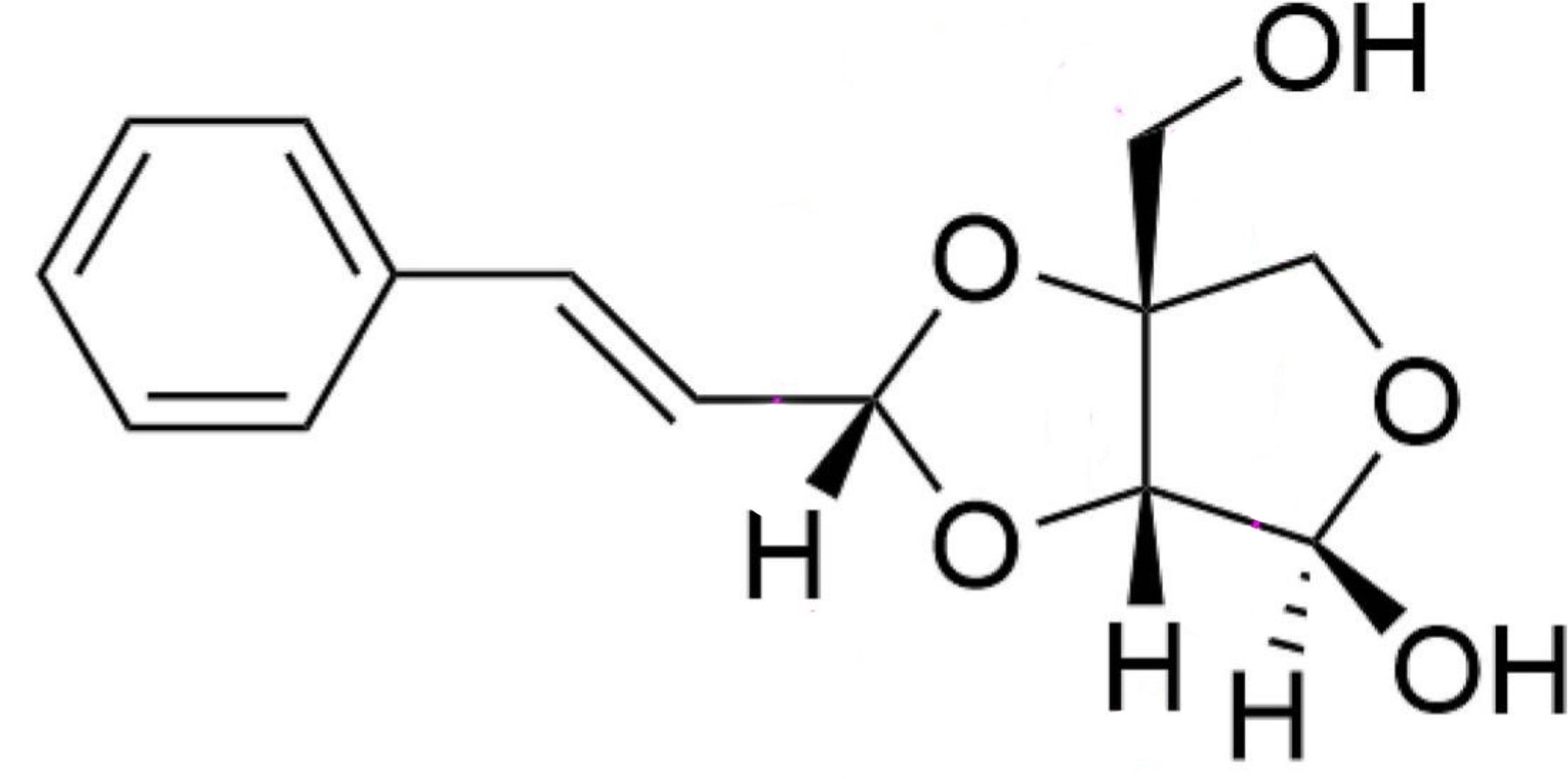 Cinnacassdiol
Cinnacassdiol
In natural phenolic lipids, the common structure of the phenolic group and the alkyl chain is altered at the level of the aromatic ring which may be a catechol or a resorcinol and of the carbon chain with variations in length and degree of unsaturation. Some parent molecules are based on a hydroquinone instead of a catechol or resorcinol.
Due to their strong amphiphilic character, phenolic lipids can incorporate into erythrocytes and liposomal membranes. The antioxidant, antigenotoxic, and cytostatic activities of these lipids have been reviewed (Stasiuk M et al., Cell Mol Life Sci 2010, 67, 841).
It has been proposed to classify the long-chain phenols into groups of phenolic acids, dihydric, and monohydric phenols. Certain plant species contain all three phenolic types, others only two and certain sources uniquely a single material. An extensive review of these lipids has been released by Tyman JHP (Chem Soc Rev 1979, 8, 499).
The simplest catechol lipid may be applied to guaiacol, chemically 2-methoxyphenol. This compound is a naturally-occurring organic lipid first isolated from guaiacum wood (Guaiacum sanctum) or creosote. It is present in wood smoke, resulting from the pyrolysis of lignin, and contributes to the flavor of many substances such as whisky and roasted coffee.
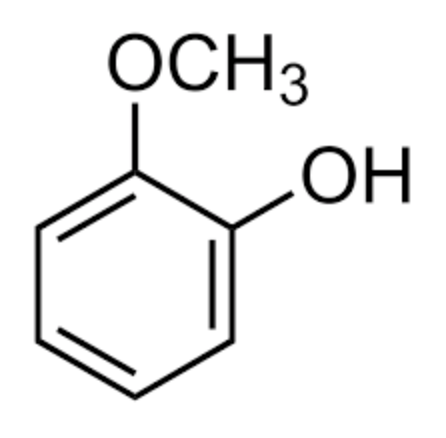 Guaiacol
Guaiacol
Guaiacol is a precursor to various very useful molecules, such as eugenol and vanillin. It is used as a chemical intermediate for the synthesis for food materials and perfurmery products, including cosmectics, personal care and industrial cleaning products.
Unexpectedly, it is also produced in the gut of desert locusts, and is one of the main components of the pheromones that cause locust swarming (Dillon RJ et al., Nature. 2000, 403 (6772), 851).
Creosol is the methylated derivative of guaiacol (4-methylguaiacol), thus a little more lipophilic than its precursor. Creosol is present in wood tars, especially pine tar, and in creosote that comes from it, but it is found in greater quantities in beech wood. It is also present with guaiacol in the pyrolysis product of guaiac and in pine resin. It is also found in vanilla pod, juniper cade (Juniperus oxycedrus), tobacco and many other plants.
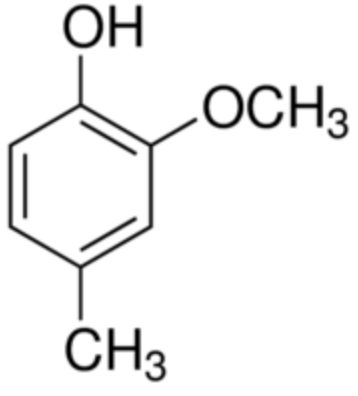 Creosol
Creosol
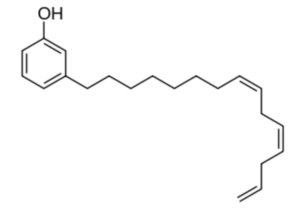
Similar phenolic lipids, but more complex with a longer carbon chain, are named Urushiol, from the Japanese word for the sap found in several parts of the lacquer tree, kiurushi (Rhus toxicodendrum, poison ivy). This name is sometimes used for all phenolic lipids.
These substances dry in air to form lustrous varnish, they are used since a long time in China and Japan for lacquering process. They bind to skin and their allergenicity increases with the unsaturation and lengthening of the side chain.
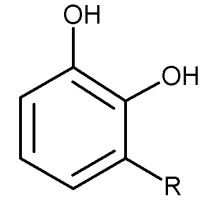
R= 13 to 17 carbon atoms, the chain having 0 to 3 double bonds
according to the plant species.
A new group of lipophilic hydroxytyrosyl derivatives with linear alkyl side chains of variable length have been synthesized from hydroxytyrosol recovered from olive oil waste waters.
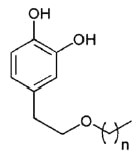
Hydroxytyrosol ether
n = 1 to 18
These compounds show strong antioxidant activity and have potential use as functional ingredients to improve the stability of foods (Pereira-Caro G et al., Food Chem 2009, 115, 86)..
Similar compounds but with an ester link between the catechol nucleus and a fatty acid (palmitic, stearic, oleic acids) have been synthesized for their lipophilic antioxidant properties. All derivatives showed better radical-scavenging capacities than did α-tocopherol and ascorbyl palmitate (Torres de Pinedo A et al., Food Chem 2007, 105, 657).
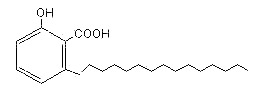
Parent compounds with an additional carboxylic group are found in cashew nut shell (pericarp) (Anacardium occidentalis) and in seeds of Ginkgo tree, the anacardic acids.
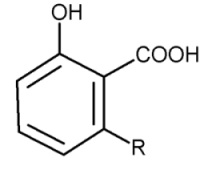
Anacardic acids
Anacardic acids form a mixture of several closely related organic compounds, each consisting of a salicylic acid substituted with an alkyl chain R (saturated or unsaturated) that has 15 or 17 carbon atoms. The composition of the mixture depends on the species of the plant. They are lethal to gram positive bacteria, the 15-carbon species with an unsaturated side chain being the most active.
Phenolic derivatives (cardanol) and resorcinolic derivatives (cardol) are present in cashew-nut oil but are mainly obtained after thermal decarboxylation of anacardic acid as byproduct of cashew nut processing. In term of physical properties, carbanol is comparable to nonylphenol. These derivatives have been used to make after polymerization surface coatings, modified rubbers, ion exchange resins, water-proofing materials, and in numerous other products. It appears a feasible alternative to petrochemical derived phenol for many applications. As an example, many projects explore its grafting onto neoprene rubber, the products being used for vulcanization.
 Cardanol
Cardanol
The most representative is ginkgolic acid (R = 15 carbon atoms) found in Ginkgo biloba extracts (pulp and leaves). All these compounds are strongly allergenic and were shown to damage DNA (a risk for cancer development) and is toxic to nerve cells (Ahlemeyer B et al., Eur J Pharmacol. 2001, 430) and the immune system. Pharmaceutical-grade Ginkgo extract must contain less than 5 parts per million of ginkgolic acid.
Ginkgolic acid
Long-chain anacardic acid derivatives, named janohigenins, were isolated from the endosperm of Ophiopogon japonicus seed, and their structures were elucidated. Janohigenins exhibit neuroprotective activity for rotenone-induced cellular damage (Ohta S et al., Phytochemistry 2021, 191,112904).
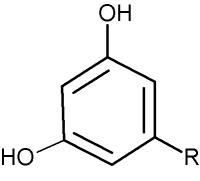
These lipids are formed of a resorcinol (1,3-dihydroxybenzene) group alkylated at the position 5 (-R) with an odd-numbered carbon chain (non-isoprenoid) of various length (C17 to C27) either saturated (alkylresorcinols) or unsaturated (alkenylresorcinols) with 1 to 6 double bonds. They are very hydrophobic molecules, especially those with the chain exceeding 11 carbon atoms.
Alkylresorcinols
They were first described Ginkgo biloba (Anderson HH et al., Proc Soc Exp Biol Med 1931, 28, 609) and later in several other Anacardiaceae such as in cashew nut shell liquid (Anacardium occidentale) (Symes WF et al., J Am Chem Soc 1953, 75, 4952), mango latex and peel. In these plants, resorcinol lipids were shown to be strong vesicants causing frequently strong allergic responses. Cardol (5-pentadecyl-1,3-resorcinol) was shown to be the strong vesicant in cashew. Cashew nuts is the main source of phenolic lipids for the synthesis of formaldehyde-polymers used in the automobile industry. Several bis-5-alkylresorcinol derivatives have been described in Proteaceae (Gadea A et al., Phytochem Rev 2022, 21, 1969–2005).
Resorcinol lipids were shown to be present not only in many higher plants but also in bacteria, in fungi, in algae, and mosses. The occurrence of various derivatives (ring or chain modified) have also been described. These amphiphilic compounds exist in a high structural diversity and can be divided into two main groups, i.e., 5-alkylresorcinols and 2-methyl-5-alkylresorcinols.
The structures of more than 100 identified natural resorcinolic lipid homologues may be found in 11 families of higher plants and have been reviewed by Kozubek et al. (Chem Rev 1999, 99, 1). Two alkyl resorcinols with strong cytotoxic activities have been isolated from rhizomes of Ardisia gigantifolia (Liu H et al., Phytochemistry 2009, 70, 773). The alkyl chain has 15 or 17 carbon atoms.
They are most common in cereal grains, e.g., rye (Secale cereale), wheat (Triticum aestivum), barley (Hordeum vulgare), and millet (Pennisetum thyphoides) (Ross AB et al., J Agric Food Chem 2003, 51, 4111). The pseudocereal quinoa (Chenopodium quinoa) has a very unique alkylresorcinol profile, consisting not only of straight-chain alkyl chains but also iso- and anteiso-branched isomers (Ross AB et al., Food Chem 2017, 220, 344).
In the major cereals, alkylresorcinols have been reported to be present in high levels (> 500 mg/g) in wheat and rye and in lower amounts in barley and millet. They are found mainly in the outer layers (bran fraction) of cereal grains. The homologue composition also varies greatly across the species; barley has the highest proportion of C25:0 (about 40%), rye has the highest proportion of C19:0 (about 30%), and wheat has the highest proportion of C21:0 (about 50%). Alkenylresorcinols are also present in cereals. Their amounts vary between 0.5% for barley, 1% for wheat, and 15% for rye. Not only the amounts but also the compositions of the specific mixtures are different. Thus, the ratio of the homologues C17:0/C21:0 is about 0.1 in wheat and 1.0 in rye, indicating that this determination may be used as good markers of these cereal products (Chen Y et al., J Agric Food Chem 2004, 52, 8242). The studies of alkylresorcinols as biomarkers for whole grains have revealed substantial variations in their pattern and homologue contents among different wheat samples, which were significantly influenced by wheat variety and cultivation location (Gao F et al., J Agric Food Chem 2025, on line November 18).
After epidemiological studies have associated the consumption of whole grains products with the decreased risk of chronic diseases (heart diseases, diabetes, some cancers), phenolic compounds found in the cereals’ bran fraction were proposed as a possible mechanism for that protection (Slavin JL et al., Am J Clin Nutr 1999, 70, 459).
5-Heptadecylresorcinol was shown to Improve aging-associated hepatic fatty acid oxidation dysfunction via regulating adipose Sirtuin 3 (Zhang K et al., Nutrients 2024, 16(7), 978). Applied research have demonstrated how alkylresorcinols from wheat bran could represent sustainable and innovative natural antioxidants for emulsion-based food (Cantele C et al., Food Chem oct 2024, 141659).
In 2019, a study examined whether plasma 3-(3,5-dihydroxyphenyl)-1-propanoic acid (DHPPA), a biomarker of whole-grain wheat and rye intake, is associated with risk of ischemic stroke. It was thus shown that higher plasma DHPPA concentrations were associated with lower risk of ischemic stroke. This finding provides further evidence to support the health benefits of whole-grain consumption (Sun T et al., Am J Clin Nutr, 2019, 109, 1).
The occurrence of alkylresorcinols in mosses and algae is poorly documented but mixtures of six 5-alkylresorcinol homologues were isolated from the microalga Apatococcus constipatus. The predominant compounds have a C19, C21, or a C23 alkyl chain (Zarnowski R et al., Phytochemistry 2000, 55, 975).
Alkylresorcinols were reported to have antiparasitic, anticancer, antifungal, antimicrobial, and antioxidant effects. Their various biological activities suggest their involvement in the regulation of cell metabolism. These activities have been comprehensively reviewed by Kozubek A et al. (Chem Rev 1999, 99,1; Cell Biol Mol Lett 2001, 6, 351). Several alkenyresorcinols have been isolated from a small herbaceous plant, Labisia pumila, used in traditional medicine in the Malay archipelago (Al-Mekhlafi NA et al., Phytochemistry 2012, 80, 42).
A specific alkylresorcinol, xenognosin, is involved in the relationships between host plants and parasite germination. These relationships have been determined mainly in studying the parasitism of cultivated plants by Striga asiatica (Fate GD t al., J Am Chem Soc 1996, 118, 11369). It seems that a methylation in C4 and C6 on the benzenic cycle is requisite for its activation. A review on the xenognosin signaling may be consulted (Keyes WJ et al., J Plant Growth Reg 2000, 19, 217).
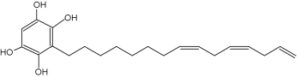
Xenognosin
Eight chlorinated alkylresorcinols, monochasiol A–H, were isolated from the fruiting bodies of Dictyostelium monochasioides. Monochasiol A selectively inhibited the concanavalin A-induced interleukin-2 production in Jurkat cells, a human T lymphocyte cell line (Kikuchi H et al., J Nat Prod 2017, 80, 2716).
Adipostatin E has been identified in the marine Streptomyces blancoensis, as a potent inhibitor of Streptococcus pneumoniae coenzyme-A biosynthesis, by targeting phosphopantothenoylcysteine synthetase), which was considered “an effective drug target” (Gómez-Rodríguez L et al., Tetrahedron Lett. 2020, 61(5), 151469).
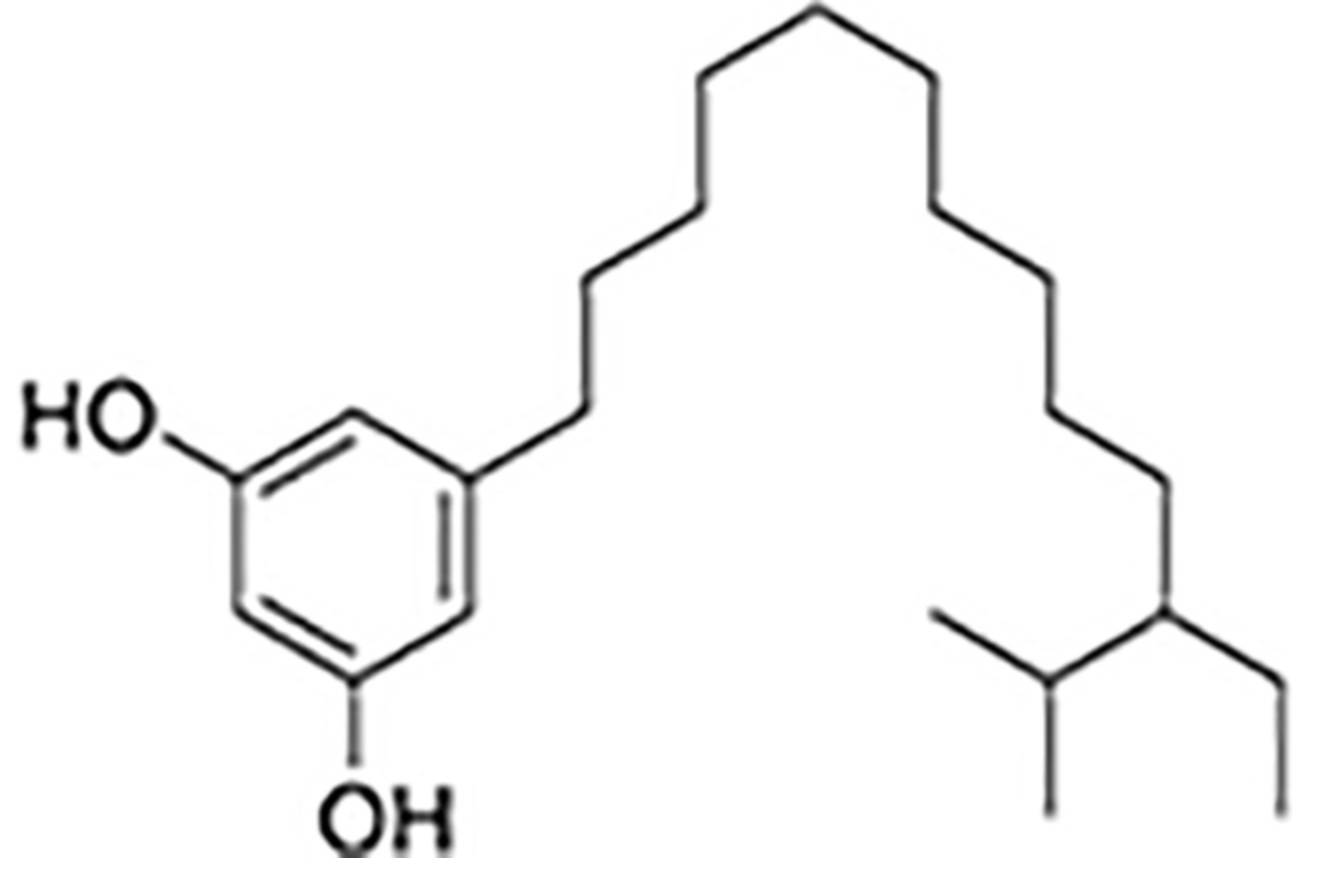 Adipostatin
Adipostatin
3,5-Dihydroxy-4-methoxybenzyl alcohol (DHMBA) may be considered as a methoxylated derivative of a resorcinol molecule.it is found in oyster extracts and is reported to have potent antioxidant activity. A study provided evidence that it could have a potential role on antiaging in brain (Chen M et al., Mol Nutr Food Res 2024, 68:e2300469). New findings have demonstrated the mechanism by which DHMBA alleviates Alzheimer’s disease and provide a new insight into its treatment (Li YF et al., Food Biosci 9 October 2024, 105259).
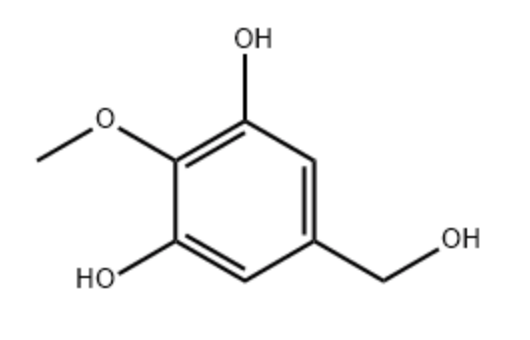 3,5-Dihydroxy-4-methoxybenzyl alcohol
3,5-Dihydroxy-4-methoxybenzyl alcohol
Cannabigerol (CBG) is composed of a resorcinol core which is substituted by a (2E)-3,7-dimethylocta-2,6-dien-1-yl group at position 2 and by a pentyl group at position 5. It is a natural product found in Cannabis sativa and Helichrysum species. It has a role as an appetite enhancer, a plant metabolite, a cannabinoid receptor agonist, an anti-inflammatory agent, an antibacterial agent, a neuroprotective agent and an antioxidant. It is considered as a phytocannabinoid.
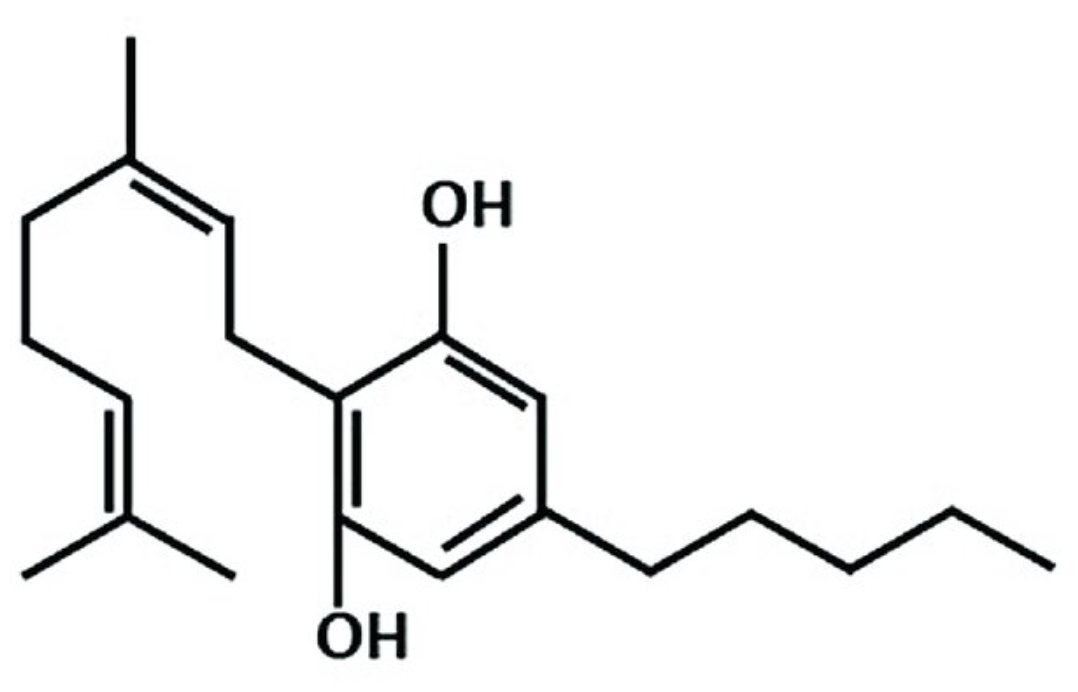 Cannabigerol
Cannabigerol
During plant growth, most of CBGl is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD).
Extensive information on these lipids may be found in the website of Dr A. Kozubek. The chemistry, bioactivity and biosynthesis of cyanobacterial alkylresorcinols have been reviewed (Martins TP et al., Nat Prod reports 2019, 36, 1437). CBGl has affinity and activity at CB1 and CB2 cannabinoid receptors in vitro. It is unique among other cannabinoids by having high affinity and activity at α2 adrenergic receptors and moderate activity at serotonin 5-HT1A receptors (Nachnani R et al., J Pharmacol Exp Ther 2021, 376, 204).
Resorcylic acid lactones are fungal polyketides that consist of a β-resorcylic acid residue (2,4-dihydroxybenzoic acid) embedded in a macrolactone ring. They exhibit a broad range of biological activities, including anticancer, antimalarial, antifungal, and immunomodulatory effects.. Examples include the mycoestrogens (and synthetic analogues) zearalenone, zearalanone, zeranol (α-zearalanol), taleranol (β-zearalanol), α-zearalenol, and β-zearalenol.
As an example, Zearalenone, also known as F-2 mycotoxin, is a resorcyclic acid lactone derived from β-resorcylic acid which is a carboxyl derivative of resorcinol (Wikipedia). Its structure being similar to estrogens, it is a potent estrogenic metabolite produced by some Fusarium and Gibberella species. It causes infertility, abortion or other breeding problems, especially in swine. There are case reports of early puberty in girls chronically exposed to zearalenone in various regions of the world.
That mycotoxin is found worldwide in a number of cereal crops, mainly in corn but also in barley, oats, rice, wheat and sorghum. Average dietary intakes of zearalenone from cereals and legumes in the GEMS/Food regional diets are 0.03 µg/kg bw/d (European diet). The total tolerable intake is 0.5 µg/kg bw/d for total intake of zearalenone and its metabolites.
The current methods used to detoxify zearalenone can be categorized into three major classes: physical, chemical, and biological methods. Among them, the biosorption method may be considered as the most economical treatment method due to its simple operation and design mode and low cost-effectiveness (Fang J et al., Food Chem 2024, 460, 140593). Biological detoxification and application of emerging control strategies of zearalenone in food have been reviewed (Yang G et al., Food Chem 2025, online 29 August, 146170).
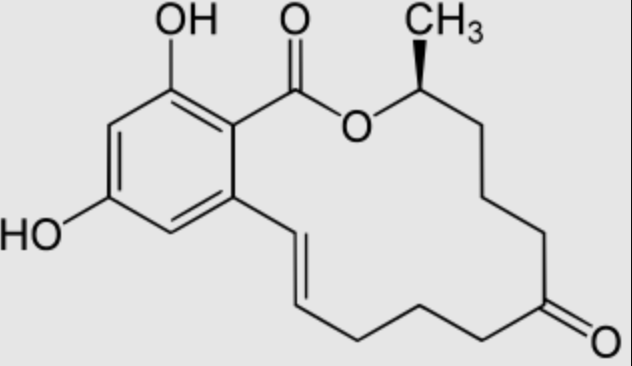 Zearalenone
Zearalenone
The chemical and biological properties of natural resorcylic acid lactones have been reviewed (Gap Y et al., Nat Prod Rep 2025, 42, 1387).
These phenolic lipids have an aromatic ring belonging to the hydroquinone group linked to a straight carbon chain.
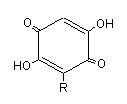
Embelin is a well known species of hydroquinone lipid which has a lateral chain (R) of 11 carbon atoms. It was characterized in a Myrsinaceae, Embelia ribes, which has antifertility properties and used as anthelminthic agent in India. Several alkyl benzoquinones have been isolated from another Myrsinaceae, Ardisia virens, which is used as a folk medicine for anti-vomiting and anti-diarrhea in Asia (Chang HS et al., Phytochemistry 2009, 70, 2064).
Sorgoleone is an allelochemical produced by several Sorghum species which is a natural herbicide repressing the growth of other plants present in their surroundings (bioherbicide) (Dayan FE, Planta 2006, 224, 339). Several analogues with various substitutions and chain lengths are found in root exudates. The major species is 2-hydroxy-5-methoxy-3-(8’,11’,14’-pentadecatriene)-1,4-benzoquinone.
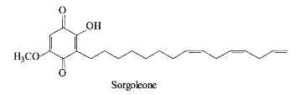
Biosynthetic studies have shown that the unusual fatty acid 16:3D9,12,15 synthesized in Sorghum roots is added to a hydroquinone, the new product being further methylated (Pan Z et al. J Biol Chem 2007, 282, 4326). Sorgoleone as an inhibitor of mitochondrial respiration and photosynthesis is extensively studied for field applications. A pertinent review on sorgoleone may be consulted for further details (Dayan FE et al., Phytochemistry 2010, 71, 1032).
An ubiquinone derivative, pseudoalteromone, has been isolated from marine Pseudoalteromonas spp., and their anti-melanogenesis activity was demonstrated (Lim SJ et al., Mar Drugs 2021, 19(11), 612).
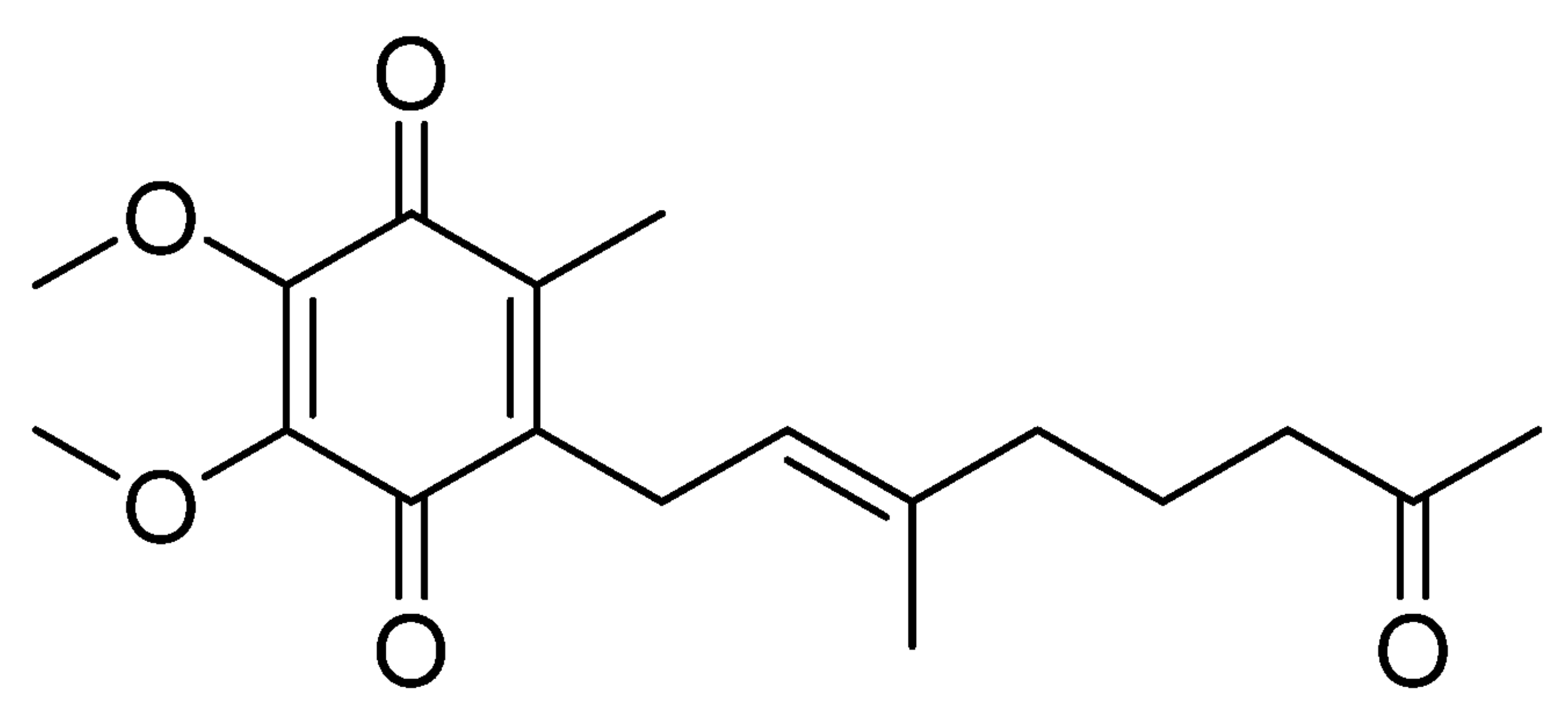 Pseudoalteromone
Pseudoalteromone
Dimeric &,4-benzoquinones derivatives, belamcandaquinones, were first described in Belamcanda chinensis plants (Fukuyama Y et al., Tetrahedron Lett 1993, 34, 7633). These compounds displayed cyclooxygenase inhibitory activity. They were later described in other plants : Ardisia punctata (Li C et al., Acta Pharmacol Sinica 2006, 41, 830) and Ardisia gigantifolia (Liu H et al., Phytochemistry 2009, 70, 773). One of these compounds is shown below.
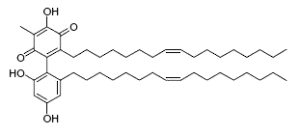
Belamcandaquinones I from Ardisia gigantifolia
PHLOROGLUCINOL LIPIDS
These lipids have a cyclic core named phloroglucinol. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol).
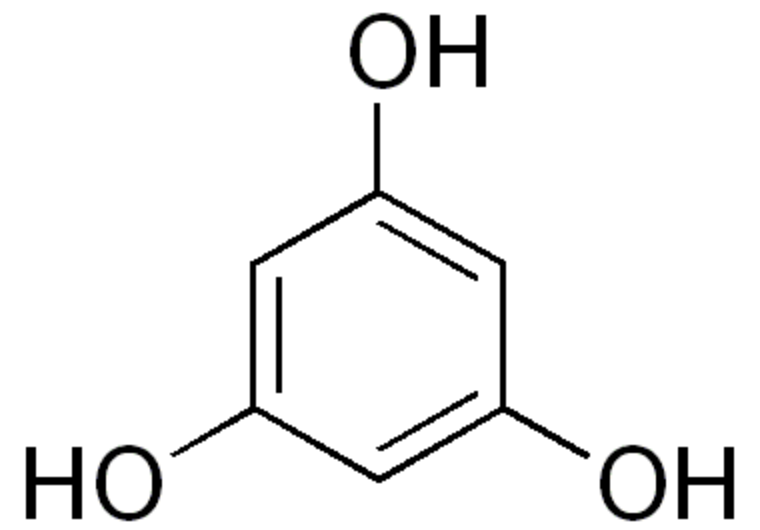
Phloroglucinol
Acylphloroglucinols have been isolated from a fern, Dryopteris crassirhizoma (Na M et al., Bioorg Med Chem Lett 2006, 16, 4738) and some have fatty acid synthase inhibitory activity.
Several phloroglucinol derivatives were also isolated from the fruit of the tropical tree, Syzygium jambos (Myrtaceae). There were named jambones (Li GQ et al., J Agric Food Chem 2015, 63, 10257). Seven acylphloroglucinol derivatives, named oblatones, were isolated from Syzygium oblatum. Their structures were determined and shown to possess an α,β-unsaturated ketone lipid chain. Some of the isolates (oblatone C) showed inhibitory effects on ATP citrate lyase in vitro (Huang JC et al., Phytochemistry 2021, 187, 112765).
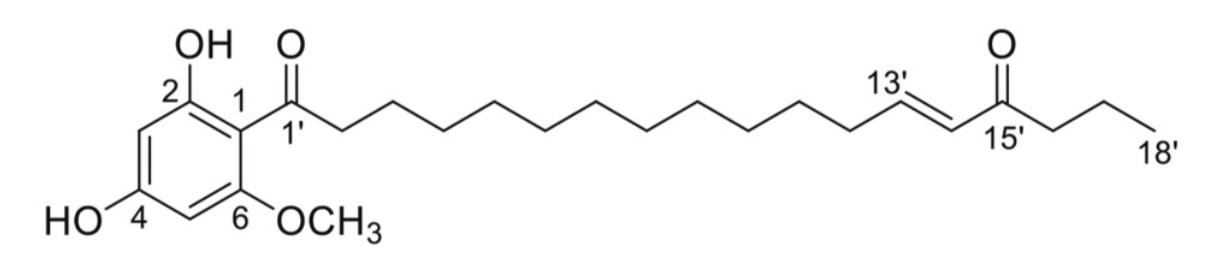
One of the oblatones (oblatone C) from S. oblatum
Phytochemical investigation of aerial parts of Helichrysum niveum using different chromatographic methods afforded the discovery of several acylphloroglucinols with excellent total antioxidant capacities which underpin new perspectives for the exploitation of these natural phenolic compounds in applications such as in the natural cosmeceutical and pharmaceutical sectors (Popoola OK et al., Molecules 2015, 20, 17309).
Dodecyl gallate is an organic compound, resulting from the esterification of gallic acid (trihydroxybenzoic acid) with dodecanol. It is used as a food additive (preservative, antioxidant) under the name E312, in particular to prevent the rancidity of fatty products. Phospholipid Complexes of dodecyl gallate was synthesized to obtain: a novel structure for controlled release of polyphenols (Wang Q et al., Eur J Lipid Sci Technol 2025, 127, e70029).
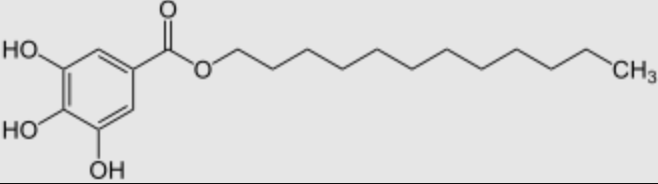 Dodecyl gallate
Dodecyl gallate
Hyperforin is a phytochemical produced by some of the members of the plant genus Hypericum, notably Hypericum perforatum (Clusiaceae) (St John’s wort). Hyperforin may be involved in the pharmacological effects of St. John’s wort, specifically in its antidepressant effects.
Hyperforin has attached increasing attention due to its multiple pharmacological activities (review in Li XX et al., Phytochemistry 2023, 206, 113526).
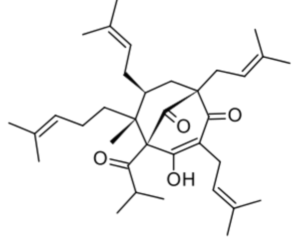
Hyperforin
.
PHENOLIPIDS OR LIPOPHENOLS
To extend the antioxidant properties of polyphenols, the natural antioxidants extracted from plants, to oils and fats, it is necessary to modify them by adding aliphatic chains to these molecules. This transformation gives rise to new artificial compounds named phenolipids.
Thus, the health benefits of phenolipids may be attributed to the characteristics of the parent polyphenolic compounds. In parallel, the lipid domains confer special physicochemical properties to the phenolipid. This transformation is mainly done through esterification or transesterification with fatty alcohols. Grafting a fatty acid acyl chain to a polyphenol modifies the antioxidant properties of the parent compound. Their biosynthesis may be performed by esterifying the -COOH or the -OH residues in the polyphenol with fatty alcohol or acid with aliphatic chains with variable length.
These phenolipids have a good metabolic stability and bioavailability due to their high lipophilicity.
Several phenolic cores have been used: gallic acid, syringic acid, vanillic acid, ferulic acid, coumaric acid, cinnamic acid, caffeic acid, naringin, rutin …
The lipophilic domain may have from one to 20 carbon atoms. Some investigations have been done with longer chains such as EPA and DHA (Oh WY et al., J Agric Food Chem 2017, 65, 8617 and Ambiguipalan P et al., Food Chem 2020, 309, 125609). It has been determined that both polyunsaturated fatty acid and isopropyl substituents bond to phloroglucinol were essential to confer the highest protection of retinal cells against the oxidative stress exerted by high doses of all‐trans‐retinal (Cubizolle A et al., J Cell Mol Med 2020, 24, 5057). These data suggest potential effects of lipophenols in the prevention of some forms of macular degeneration.
A very comprehensive review has been written on that class of molecules (Arzola-Rodríguez SI et al., Biomolecules 2022, 12, 1897).
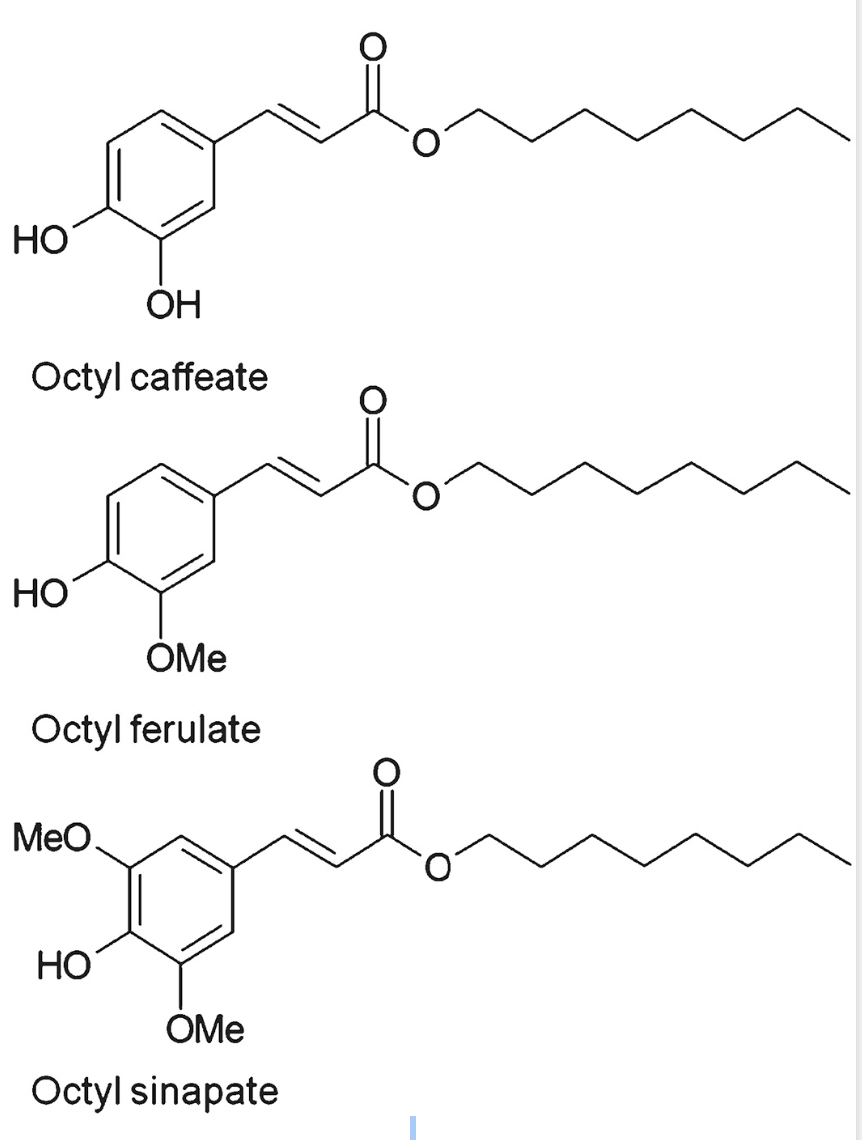
Some phenolipids used to enhance antioxidant capacity
of rapeseed–linseed oil mixture (Szydłowska-Czerniak A)
Chicoric acid (also known as cichoric acid) occurs in a variety of plant species. It is a derivative of both caffeic acid and tartaric acid. It was first been isolated from Cichorium intybus (chicory) but also occurs in significant amounts in Echinacea purpurea, Taraxacum monogolicum (dandelion) leaves, Ocimum basilicum (basil), Melissa officinalis, and some aquatic plants (Wang A et al., Nutrients 2025, 17, 3769). It has been widely used in food, medicine, animal husbandry, and other commercial products because of its significant pharmacological activities. Growing evidences from modern pharmacological research demonstrate the notable anticancer effects of dandelion (Yang K et al., Exp Ther Med 2024, 27, 256). A review of the natural occurrence, chemical synthesis, biosynthesis, and bioactive effects of chicoric acid may be consulted (Yang M et al., Front Chem 23 June 2022, 10).
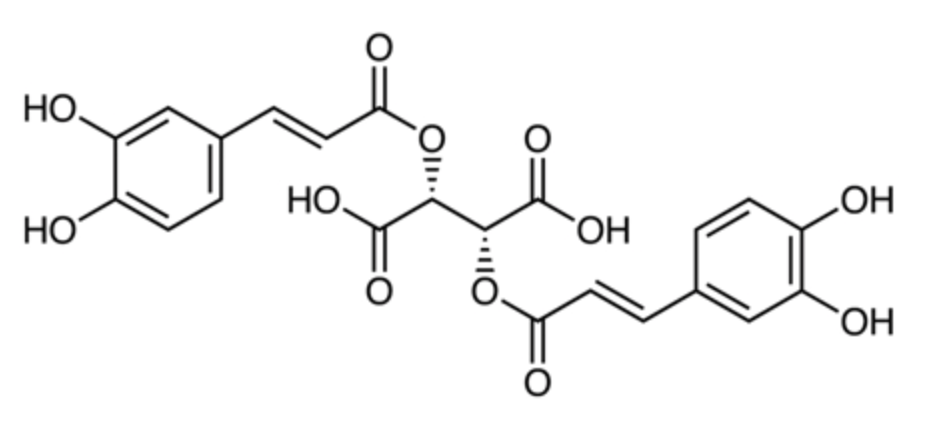 Chicoric acid
Chicoric acid
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire