
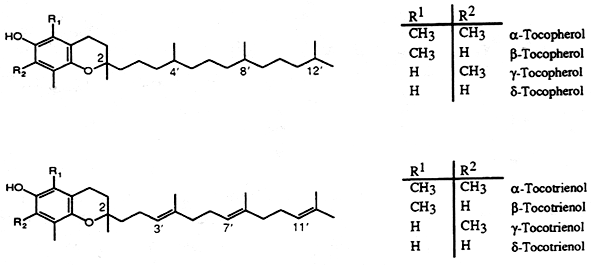
The most active form of vitamin E, α-tocopherol, is a 6-hydroxychroman derivative (Chromane or benzodihydropyran), a heterocyclic chemical compound with the chemical formula C9H10O) with methyl groups in position 2,5,7, and 8 and a phytyl side chain attached at carbon 2. There are three groups of vitamin E : tocopherols, tocotrienols and tocomonoenol. Tocopherols are composed of four known forms with a saturated phytol side chain : the α-, β-, γ-, and δ-tocopherols, the tocotrienols are composed of six forms with 3 double bonds in the side chain : α-, β-, γ-, δ-tocotrienols and desmehyl-tocotrienols, and the tocomonoenols composed of two known forms with one double bond in the side chain : α– and δ-tocomonoenols.
The term “vitamin E” should be used for all tocopherol, tocotrienol and tocomonoenol derivatives exhibiting the biological activity of d-α-tocopherol. The term “tocopherol” should be used for all methyl tocols. Since tocotrienols have some vitamin E activity, “tocopherol” is not synonymous with “vitamin E”.
A scientific debate is opened on the change the nomenclature of Vitamin E. As only RRR-α-tocopherol has been shown to protect against a deficiency disease in humans, the attributes of vitamin have to be uniquely restricted to RRR-α-tocopherol. Other vitamin analogues should be described only by their chemical names (AZZI A et al., Free Rad Biol Med 2023, 207, 178).
It seems that δ-tocotrienol is the earliest member of the group to be formed in plants, methylation leading to the other tocotrienols and hydrogenation producing the respective tocopherols. Phytyl diphosphate is the prenyl precursor for tocopherol side chain biosynthesis. Based on genetic evidence, phytyl diphosphate is supplied to the tocopherol biosynthetic pathway primarily by chlorophyll degradation and sequential phytol phosphorylation (Zhang W et al., Plant Physiol 2014, 166, 70).
Natural α-tocopherol, termed d-α-tocopherol, may be described chemically as 2R-(4’R,8’R)-5,7,8-trimethyltocol, the term tocol being the name for the 2-ring structure (benzopyran-6ol) basic to all vitamin E compounds.
The natural tocotrienols exist as D-stereoisomers: D-α-tocotrienol, D-β-tocotrienol, D-γ-tocotrienol and D-δ-tocotrienol. In reference to the geometric configuration of the double bonds of the side chain, the natural tocotrienols are designated as E. D-α-Tocotrienol is also known as 2R,3’E,7’E-α-tocotrienol and is abbreviated α-T3; D-β-tocotrienol is known as 2R,3’E,7’E-β-tocotrienol and abbreviated β-T3; D-γ-tocotrienol is known as 2R,3’E,7’E-γ-tocotrienol and abbreviated γ-T3; δ-tocotrienol is known as 2R,3’E,7’E-δ-tocotrienol and abbreviated δ-T3.
Two novel tocotrienols were isolated from stabilized and heated rice bran (Qureshi AA et al., J Agric Food Chem 2000, 48, 3130). They were named desmethyl- and didesmethyl-tocotrienols as they have only one or no methyl group on the 6-hydroxychromane nucleus, respectively. It was shown that they have much greater antioxidant, hypocholesterolemic and antitumor properties than the other components of vitamin E. Furthermore, one of them (didesmethyl-tocotrienol) was shown to efficiently induce a reduction of atherosclerotic lesions in mice (Qureshi AA et al., J Nutr 2001, 131, 2606).
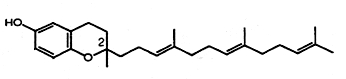
Desmethyl-tocotrienol
(didesmethyl-tocotrienol has no methyl group on carbon-2)
Homologues of γ-tocotrienol, named plastochromanol, have been described in vegetal tissues, such as leaves and seeds. The first species was discovered in the leaves of Hevea brasiliensis in 1965 by thin layer chromatography and chemical identification (Whittle KJ et al., Biochem J 1965, 98, 17c). These homologues have the same chromanol nucleus as γ-tocotrienol but have a longer terpenoid chain, most frequently formed of 8 isoprenoid units (plastochromanol-8).
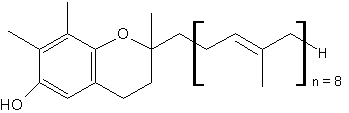
Plastochromanol-8
It is found at the highest amounts in flax and rape seed oils, as well as in minor amounts in seed oils of camelina, maize, hemp, many Vietnamese plants, salvia, black pine and many others (see: Szymañska R et al. Acta Biochim Polonica 2010, 57, 105). Its function in seeds seems to be similar to that of tocopherols, i.e., antioxidant action but the literature data on that subject is very limited (Olejnik et al., Nahrung 1997, 41, 101). It has been shown to be a potent antioxidant (Olejnik D et al., Mol Nutr 2006, 14, 101) and even essential during seed desiccation and quiescence (Mene-Saffrane L et al., PNAS 2010, 107, 17815). It has been shown also to be an efficient singlet oxygen quencher in vitro, being even more active than γ-tocopherol or γ-tocotrienol in hydrophobic solvents (Gruszka J et al., Free Radic Biol Med 2008, 45, 920).
The isolation of plastochromanol-8 from flaxseed oil has indicated the additional presence of lower shares of two previously unknown homologues, plastochromanol-7 (PC-7) and plastochromanol-9 (PC-9), which feature seven and nine isoprenoid units respectively on the γ-chromanol backbone (Hammerschick T et al., Mol Nutr Food Res 2023, 67, 2300333).
γ-Tocotrienol-9 has been identified in lipid extracts of yam tuber (Dioscorea alata) (Cheng WY et al., J. Agric. Food Chem. 2007, 55, 7350). This isoform is structurally similar to γ-tocotrienol, except that the phytyl side chain is much longer.
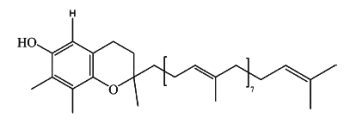
γ-Tocopherol-9
An analysis of tocotrienol accumulation in plants has revealed the presence of tocotrienols in several non-photosynthetic tissues and organs (seeds, fruit, latex) (Horvath G et al., Phytochemistry 2006, 67, 1185). Excepted coleoptiles, no tocotrienols could be detected in mature photosynthetic tissues.
A comprehensive and systematic review on the structurally diverse toco-chromanols and toco-chromenols found in photosynthetic organisms, including marine organisms, and as metabolic intermediates in animals may be consulted (Birringer M et al., RSC Adv 2018, 8, 4803).
– A new vitamin E constituent, α-tocomonoenol, was discovered in palm oil (Matsumoto A et al., J Jap Oil Chem Soc 1995, 44, 593) and was attributed as a biosynthetic intermediate along the reductive pathway from tocotrienols to tocopherols.
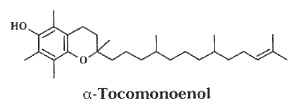
Later, an isomeric form of that compound was isolated from salmon eggs and named “marine-derived tocopherol”, it was shown to have identical antioxidant activity as does α-tocopherol (Yamamoto Y et al., J Nat Prod 1999, 62, 1685). It was also shown that it is broadly distributed in the tissue of marine fish, its content being greater in cold-water fish than in tropical fish (Yamamoto Y et al., PNAS 2001, 98, 13144).
In 2009, another form was isolated from Actinidia chinensis (kiwi) fruits and its structure elucidated as δ-tocomonoenol (Fiorentino A et al., Food Chem 2009, 115, 187).
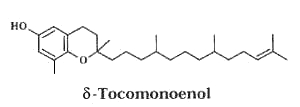
This compound was found four times more concentrated in the fruit peel than in the pulp (0.64 vs 2.49 mg/100g fresh weight). The radical-scavenging capacities of δ-tocomonoenol and d–tocopherol appear quite similar but slightly lower than that of α-tocopherol.
– Various compounds of the chromene class have been isolated from a wide variety of organisms (coelenterates, macroalgae, sponges, and tunicates). They possess a polyprenyl chain attached to a hydroquinone or similar aromatic ring moiety, they are classified among the meroterpenoids. These compounds are secondary metabolites formed by mixed biosynthetic pathways which are produced in part from a terpenoid co-substrate. These hybrid compounds are widely produced by bacteria, algae, plants, and animals. A great chemical diversity is generated via a combination of terpenoid scaffolds with polyketides, alkaloids, phenols, and amino acids. A review deals with the isolation, chemical diversity, and biological effects of numerous meroterpenoids from natural sources (Nazir M et al., Biomolecules 2021, 11, 957). Most of the meroterpenoids possess antimicrobial, cytotoxic, antioxidant, anti-inflammatory, antiviral, enzyme inhibitory, and immunosupressive effects.
Merochlorins are chlorinated merochlorins, they are biosynthesized from tetrahydroxynaphthalene linked to a modified C15 isoprene unit (Kaysser L et al., J Am Chem Soc 2012, 134, 11988), several displaying strong antibacterial activities (Ryu MJ et al., Mar Drugs 2021, 19, 618).
Several chromenes were described in brown algae such as Sargassum siliquastrum (Jang KH et al., J Nat Prod 2005, 68, 716). The extensive analysis revealed detailed structural differences between the sargachromanols. One of these structures (Sargochromanol A) has a nucleus similar to that of δ-tocotrienol and a diprenyl chain substituted with a terminal aldehyde group (see below). Several compounds exhibited significant antioxidant activity.
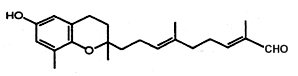
Sargochromanol A
Other forms, present in Sargassum fallax, have a more complex polyprenyl chain (diterpenoid) substituted with various chemical groups (hydroxyl, ethyl, carboxyl) (Reddy P et al., Phytochemistry 2009, 70, 250).
Similar to their chromanol counterparts, sargachromenols were named after the brown algae species Sargassum serratifolium, from which they have been isolated first (Kusumi T. et al., Chem Lett 1979, 8, 277). chromenols consist of a 2-methyl-2H-chromen-6-ol core that is associated with a side-chain with varying chain length and varying chemical modifications, leading to high structural diversity. Just like sargachromanols, sargachromenols comprise a molecule class of high structural diversity due to different side-chain modifications. The multitude of these compounds can be obtained from photosynthetic organisms like plants, algae, cyanobacteria, fungi, corals, sponges, and tunicates (Birringer M et al., RSC Adv 2018, 8, 4803). Brown algae from the Sargassaceae family have been used in traditional Asian medicine as well as in health promoting diets, revealing a variety of biological functions (Wallert M. et al., Front Pharmacol 2020, 11, 362). Sargachromenol is now described to have potent anti-inflammatory effects. It has been demonstrated that it significantly downregulates the particulate matter-induced proinflammatory cytokines, Prostaglandin E2, and Nitric Oxide secretion via blocking downstream activation of Toll-like receptor-mediated nuclear factor kappa B and MAPKs phosphorylation. Thus, Sargachromenol appears to be a potential candidate for innovation in various fields including pharmaceuticals, cosmeceuticals, and functional food. The current knowledge on the biological functions of chromenol structures is very sparse (Wallert M et al., Front Pharmacol. 2020; 11: 362),
HISTORY : Vitamin E was discovered in 1922 when Evans HM et al. (Science 1922, 56, 650) described a “substance X” that was essential to maintain rat fertility. After obtaining similar results, Sure B called the substance “vitamin E” because vitamins A, B, C, and D were already known (Sure B, J Biol Chem 1924, 58, 693).
Pure α-tocopherol was isolated from wheat-germ oil in 1936 (Evans HM et al., J Biol Chem 1936, 113, 319). Evans et al. called for the first time the isolated substance “tocopherol”, a name derived from the ancient Greek word phero, “to bring” and the word tocos, meaning “childbirth”.
By 1931, Cummings MJ et al. suggested that vegetable oils rich in vitamin E could prevent the loss of vitamin A in mixed diets by protecting it from oxidation and suggested that vitamin E had “anti-oxidant activity” (J Nutr 1931, 3, 421). Later, in 1937, Olcott HS et al determined that all tocopherols are effective antioxidants (J Am Chem Soc 1937, 59, 1008).
The correct structure of α-tocopherol was given in 1938 (Fernholz E, J Am Chem Soc 1938, 60, 700), and its first synthesis (all-racemic form) realized the same year (Karrer P et al., Helv Chim Acta 1938, 21, 820).
Tocotrienols were prepared for the first time in 1963 (Schudel P et al., Helv Chim Acta 1963, 46, 2517) and synthesized in 1976 (Scott JW et al.,Helv Chim Acta 1976, 59, 290).
A comprehensive history of the discovery of vitamin E may be found in a paper by Evans HM (Vitam Horm 1962, 20, 379) and Wolf G (J Nutr 2005, 135, 363). The history of human vitamin E deficiency and the evidence as to why vitamin E should be defined as 2R-α-tocopherol have been reviewed (Traber MG, Free Rad Biol Med 2024, 213, 285).
BIOLOGY :
Plants: α-Tocopherol is universally distributed in the plant kingdom and is the predominant vitamin E form in photosynthetic tissues, γ-tocopherol and tocotrienols predominate in the seeds of several dicots and monocots, respectively. Due to their capacity to quench and scavenge singlet oxygen (1O2) and regulate the extent of lipid peroxidation in chloroplasts, some vitamin E forms (α-tocopherol in particular) have been shown to play an antioxidant role not only in leaves but also in some fruits and flowers. Vitamin E, and α-tocopherol in particular, appear to be essential for plant development and help to fight against a number of environmental stresses. The biological significance of findings in relation to these aspects and to other key achievements made in this field have ben extensively reviewed (Munoz P et al., Trends Plant Sci 2019, 24, 1040).
Animals: Humans and animals are unable to synthesize vitamin E, they must obtain it from plant sources. Attempts to characterize symptoms of human vitamin E deficiency began In the 1950s when Horwitt MK induced vitamin E deficiency in men by providing them a diet low in vitamin E for 6 years (Horwitt MK et al., Am J Clin Nutr. 1956, 4, :408).
Whereas various homologues are found in plants, only α-tocopherol and a lower proportion of γ-tocopherol are present in human and animal tissues including blood. Enrichment of the diet is commonly performed with synthetic all-rac-α-tocopherol acetate. The biological activities vary depending on the vitamer and the test chosen, they range from 100% for α-tocopherol to 30% for γ-tocopherol and 1.4% for δ-tocopherol. It must be noticed that γ-tocopherol was found to be more potent than α-tocopherol in its interaction with reactive nitrogen oxide species (Cooney RV et al., Proc Natl Acad Sci USA 1993, 90, 1771). Several studies indicate that γ-tocopherol may be important to human health and that it possesses unique features that distinguish it from α-tocopherol. γ-Tocopherol, which is the major form of vitamin E in many plant seeds, appears to be a more efficient antioxidant (trapping of electrophilic species) for the lipid phase than α-tocopherol. Human and animal studies indicate that plasma concentrations of γ-tocopherol are inversely associated with the incidence of cardiovascular disease and cancer (Jiang Q et al., Am J Clin Nutr 2001, 74, 714). These properties are likely related to the anti-inflammatory activity of tocopherol and its major metabolite (α-CEHC) linked to inhibition of cyclooxygenase-2 (COX-2) (Jiang Q et al., PNAS 2000, 97, 11494; Jiang Q et al., FASEB J 2003, 17, 816). It has been demonstrated that γ-tocopherol induces cell death in a prostate cancer cell line by interrupting de novo synthesis of sphingolipids (Jiang Q et al., PNAS 2004, 101, 17825). The current knowledge on the anti-inflammatory properties of α-tocopherol and γ-tocopherol, the two major forms of vitamin E in humans, have been reviewed (Reiter E et al., Mol Aspects Med 2007, 28, 668). The molecular mechanisms that may be responsible for this effect are discussed.
From a physiological point of vue, it has been shown that vitamin E deficiency in children was associated with symptoms secondary to fat malabsorption and may lead to peripheral neuropathy and increased erythrocyte haemolysis. High proportions of vitamin E deficiency provide evidence that this nutritional deficiency is a public health problem in children and still little addressed in the international scientific literature (de Castro Lobo LM et al., Nutr Res Rev 2023, 36, 392).
The various forms and metabolites of vitamin E have been reviewed with respect to their metabolism, anti-inflammatory effects and mechanisms, and in vivo efficacy in preclinical models as well as human clinical intervention studies (Jiang Q, Free Rad Biol Med 2014, 72, 76).
A report on selected chromanols and chromenols based on the availability of data on signaling pathways involved in inflammation, apoptosis, cell proliferation, and carcinogenesis has been published (Wallert M et al., Front Pharmacol. 2020; 11: 362).
It has been shown that high plasma α-tocopherol concentrationa had protective effect against diabetes incidence among persons not taking vitamin E supplements. The biologic mechanism for such a protective effect could involve improvement in either insulin sensitivity, insulin secretion or both (Costacou T et al., Diabetes Obesity Metabol 2008, 10, 223). The links between oxidative stress and diabetes mellitus have been reviewed (Yaribeygi H et al., Oxidative Med Cell Longevity 2020, ID 8609213).
Some studies have concluded that vitamin E can play a protective role in neurodegeneration with respect to diseases such as Alzheimer’s disease, Parkinson’s disease, stroke and amyotrophic lateral sclerosis. Vitamin E supplementation was also associated with risk factors for some neurodegenerative diseases (Icer M et al., Acta Neurobiol Exp 2021, 81, 1).
The ability of vitamin E to modulate signal transduction and gene expression has been observed in numerous studies; however, the detailed molecular mechanisms involved are often not clear. Vitamin E modulates the activity of several enzymes involved in signal transduction (review in : Zingg JM, Mol Aspects Med 2007, 28, 481).
Vitamin E and K interactions have been recognized for over 50 years, yet the mechanisms for this interaction are unknown (Traber MG, Nutr Rev 2008, 66, 624). High vitamin E intakes in rats caused increased bleeding which could be reversed by increased vitamin K administration. Reports from the Women’s Health Study demonstrate that vitamin E supplements decrease the risk of mortality from thromboembolism, α-tocopherol decreasing the tendency for blood to clot in normal healthy women.
The biopotency of α-tocotrienol was found to be about 30% but, recently, it was found to be a better antioxidant than α-tocopherol (Suzuki YJ et al., Biochemistry 1993, 32, 10692). The interest in tocotrienol as a hypocholesterolemic compound began in 1986 (Qureshi AA et al. J Biol Chem 1986, 261, 10544), several subsequent studies confirmed this observation (Theriault A et al., Clin Biochem 1999, 32, 309). A review summarizing the main antioxidant and nonantioxidant effects of tocotrienols and their potential as health-maintaining compounds was released by Schaffer S et al. (J Nutr 2005, 135, 151) and by Nesaretnam K et al. (Eur J Lipid Sci Technol 2007, 109, 445). Actually, all the biological activity of vitamin E is seen and understood in the light of protection of membrane fatty acids against any oxidative attack (Traber MG et al., Free Rad Biol Med 2007, 43, 4). The tocotrienol forms of natural vitamin E possesses powerful hypocholesterolemic, anti-cancer and neuroprotective properties that are often not exhibited by tocopherols. The current state of knowledge has been extensively reviewed (Sen CK et al., Mol Aspects Med 2007, 28, 692. Brigelius-Flohe R, Free Rad Biol Med 2009, 46, 543).
Experiments with endothelial cells from human umbilical vein have demonstrated that γ-tocotrienol is a potential chemopreventive agent via antiangiogenesis (Li Y et al., J Nutr Biochem 2011, 22, 1127). New advances suggesting that tocotrienols may be used for health improvement or therapeutic pruposes have been reviewed (Wong R et al., Nutr Rev 2012, 70, 483). It has been demonstrated that γ-tocotrienol attenuates oxidative stress and preserves mitochondrial function in inflammation-induced muscle atrophy (Chung JY et al., Redox Biol 2025, 87, 103874).
In 2014, Yamasaki M. et al., demonstrated that δ-tocotrienol potently induces apoptosis in T-cell leukemia cells via the depletion of intracellular squalene, showing that this compound could be considered as a therapeutic agent in patients with leukemia or lymphoma (Yamasaki M et al., Food & Function 2014, 5, 2842-9).
Molecular mechanism of tocotrienol-mediated anticancer properties has been reviewed including the involvement of endoplasmic reticulum stress and unfolded protein response (Pang KL et al., Nutrients 2023, 15, 1854).
Tocotrienol-rich fraction has recently been reported to demonstrate promising neuroprotective properties. However, it remains to be fully determined how it confers protection in the brain. A review aimed to explore and understand the intricate role of tocotrienols in promoting and preserving neuronal well-being (Yunita E et al., Int J Mol Sci 2025, 26, 7691).
An in-depth review of the pharmacology, metabolism, toxicology and biosafety aspects of tocotrienols may be consulted (Ahsan H et al., Nutr Metab 2014, 11(1):52).
One of the most surprising findings of a study of estrogenic compounds in yam tuber was that RRR-α-tocopherol, the most potent and biologically important isomer of vitamin E, displayed potent activation of estrogenic receptors (Cheng WY et al., J. Agric. Food Chem. 2007, 55, 7350). These results partly provide evidence for the beneficial effect of yam for menopausal women.
To know more about vitamin E antioxidant properties, consult the VERIS web site.
Up to 1957 an International Unit was used and defined as the vitamin E activity of 1 mg of α-tocopherol acetate (the average amount of the vitamin required to prevent gestation-resorption in rats deprived of vitamin E). Now, owing to the knowledge of the molecular purity and stereochemical composition of preparations of α-tocopherol, an international reference is no longer used.
DISTRIBUTION : Vitamin E is mainly present in oils of seeds. Alfalfa (5 mg/100 g), corn (15 mg/100 g) and soybean (110 mg/100 g) represent the major sources for animals. While tocopherols are generally present in nuts and common vegetable oils, tocotrienols are concentrated in several plant sources such as cereal grains (rye, barley, oat) and certain oils (palm oil, rice bran oil). In human nutrition, the major sources are salad oil, dressings, shortenings and margarines.
Content in vitamin E components of various vegetal oils (mg/100 g)
|
Oil |
α-T |
β-T |
γ-T |
δ-T |
α-T3 |
β-T3 |
γ-T3 |
| Sunflower |
69 |
3 |
– |
– |
– |
– |
– |
| Peanut |
18 |
10 |
22 |
– |
– |
– |
– |
| Rapeseed |
26 |
– |
36 |
1 |
– |
– |
– |
| Soybean |
11 |
3 |
74 |
36 |
– |
– |
– |
| Corn |
20 |
1 |
121 |
4 |
– |
– |
– |
| Olive |
8 |
2 |
2 |
– |
– |
– |
– |
| Wheat germ |
124 |
53 |
18 |
– |
4 |
9 |
– |
| Rice bran | 55 | 3 | 13 | 0.4 | 43 | 61 | 2 |
| Grape seed |
13 |
2 |
9 |
– |
7 |
– |
9 |
| Coconut | 0.5 | – | – | 0.6 | 0.5 | 0.1 | 0.9 |
| Palm |
9 |
– |
2 |
– |
12 |
– |
32 |
|
Cereal Species |
α-T |
β-T |
γ-T |
δ-T |
α-T3 |
β-T3 |
γ-T3 |
α-T equivalents |
| Oat |
1.4 |
0.3 |
0.04 |
– |
5.6 |
0.5 |
– |
3.4 |
| Wheat |
1.6 |
0.9 |
– |
– |
0.6 |
4.2 |
– |
2.4 |
| Corn |
0.4 |
0.02 |
4.5 |
0.04 |
0.5 |
– |
1.1 |
1 |
| Barley |
0.9 |
0.1 |
0.6 |
0.07 |
4 |
0.9 |
1 |
2.3 |
T: tocopherol, T3: tocotrienol, all are expressed in mg/100 g
Tocopherol and tocotrienol contents of several vegetable oils and industrial fats may be found in an original article (Schwartz H et al., J Food Comp Anal 2008, 21, 152). The presence of vitamin E derivatives has been also described in less widespread plant oils (Trela A et al., Food Chem 2019, 296, 160). The highest tocopherol contents were in pomegranate, wheat germ and raspberry seed oils. In general, γ-tocopherol was the predominant tocopherol homologue. The highest concentration of tocotrienols was in coriander seed oil. Plastochromanol-8 was present in most of the oils, but wheat germ oil was the richest source.
The vitamin E requirement for human adults is about 15 mg per day a-tocopherol equivalents. This amount was assessed with the use of a diet fortified with deuterium-labeled α-tocopheryl acetate, taking into account an estimated 33% absorption (Bruno RS et al., Am J Clin Nutr 2006, 83, 299).
European legislation for dietary additives accepts 4 types of tocopherols:
– E 306: natural extract enriched in tocopherols (more than 34%), added to food at a concentration from 200mg/Kg up to 1g/Kg.
– E 307: synthetic dl-α-tocopherol
– E 308: synthetic dl-γ-tocopherol
– E 309: synthetic dl-δ-tocopherol
– Ascorbic acid palmitate (E 304) is also used as food additive.
The global annual production of vitamin E exceeds 20000 tons and synthetic vitamin E represents up to 90% of the total production. The predominant amount is used for animal feeding and about a quarter is used for human applications.
The practical challenge is to keep α-tocopherol stable until use. The most common approach is to use the ester a-tocopheryl acetate which needs to be cleaved in the digestive tract to recover the antioxidant properties of a-tocopherol. A second approach is phosphorylation. Evidence has been found for tocopherol phosphate in common foods as well as present in humans, indicating that phosphorylation of tocopherol is a natural process. The transfer of a phosphoryl group (-PO3H2) to the hydroxyl group on the chroman nucleus was shown to have a potent antioxidant effect (Rezk B et al., Biochim Biophys Acta 2004, 1683, 16). It was demonstrated that the new compound acts as a detergent forming a barrier which may inhibit the transfer of radicals from a substrate to another. This new mechanism may form the basis for a new class of antioxidant. It was also reported that pretreatment of cultured mouse skin with tocopherol phosphate provided significant protection greater than the acetate derivative against UV-B-induced skin damage characterized sunburn cell formation, and DNA degradation (Nakayama et al. J. Invest. Dermatol 2003, 121, 406).
α-Tocopherol phosphate seems now to be an ideal candidate for a number of cellular functions, such as intracellular transport, cell proliferation and inflammation (Munteanu A et al., Biochem Biophys Res Commun 2004, 318, 311). Several hypotheses have been made for its possible roles in cellular signaling (Negis Y et al., IUBMB Life 2005, 57, 23). It has been even proposed that at physiological concentrations a-tocopherol may act mainly as a ligand of not yet identified specific proteins and not as an antioxidant (Azzi A, Free Rad Biol Med 2007, 43, 16). An important role in cell signaling for α-tocopheryl phosphate has also been hypothesized.
Cycloaddition products of α-tocopherol with carotenoids have been described. They were named pittosporumxanthins.
![]()
The natural metabolites of α– and γ-tocopherol have been also determined. It was demonstrated that an excess of ingested tocopherols leads to an excretion of parent molecules mainly as glucuronide or sulfate conjugates in the urine (Traber MG et al., FEBS Lett 1998, 437, 145; Stahl W et al., Anal Biochem 1999, 275, 254). These molecules have the common methyl hydroxychroman nucleus but with a short side chain (3 carbon atoms) with a carboxyl group instead of a phytyl tail (see below). α-Tocopherol is thus oxidized by the cytochrome P450 w-hydroxylase pathway which leads by a stepwise removal of two- or three-carbon moieties on the phytyl chain to the transformation into 2,5,7,8-tetramethyl-2-(2′-carboxy-ethyl)-6-hydroxychroman (α-CEHC) (Sontag TJ et al., J Biol Chem 2002, 277, 25290). α-CEHC has been previously discovered as a urinary metabolite and proposed as an indicator of the body vitamin E status (Schultz M et al., Am J Clin Nutr 1995, 62, 1527S). This P450 enzyme system is, to date, the only catabolic pathway which acts on vitamin E, some differences being observed according to the oxidized substrate (Sontag TJ et al., J Lipid Res 2007, 48, 1090).
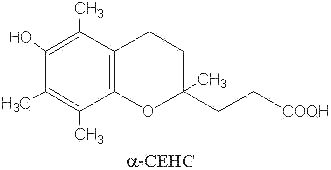
The activities of these metabolites are likely not related to their antioxidant behavior but rather to anti-inflammatory, antineoplastic and natriuretic properties (Hensley K et al., Free Rad Biol Med 2004, 36, 1).
Hydroxychromanols with a phytyl chain of 3, 5 or 9 carbon atoms were shown to be produced from tocopherols and tocotrienols in various cancerous cell types (from lung or liver) (You CS et al., J Nutr 2005, 135, 227).
γ-Tocopherol is metabolized by cytochrome P450-mediated w-hydroxylation and oxidation of the 13′-carbon on the hydrophobic chain to generate 13′-carboxychromanol (Jiang Q et al., Am J Clin Nutr 2001, 74, 714). That metabolite, with its parent compound 9′-carboxychromanol, were shown to inhibit cyclooxygenase (COX-1 and 2), property which may serve as anti-inflammation and anticancer agents (Jiang Q et al., PNAS 2008, 105, 20464). It has been shown that 13′-carboxychromanol, a long-chain metabolite of d-tocopherol, suppressed the cell-generation of the leukotriene LTB4 by blocking calcium influx, with an IC 50 of 4–7 µM, and potently inhibited human recombinant 5-LOX activity, with an IC 50 of 0.5–1 µM (Jiang Z et al., J Immunol 2011, 186, 1173). These activities provide potential molecular bases for the differential anti-inflammatory effects of vitamin E forms in vivo.
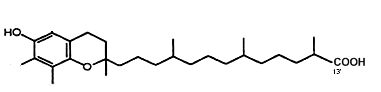
13′-carboxychromanol
α-13′-Carboxychromanol has been detected in human serum, providing evidence for its systemic bioavailability (Wallert M et al., Free Rad Biol Med 2014, 68, 43). Furthermore, that metabolite inhibits macrophage foam cell formation in vitro. A study has demonstrated that various 13′-carboxychromanols have anti-inflammatory and anticancer activities and that they may contribute to in vivo anticancer effect of vitamin E forms, so they are promising novel cancer prevention agents (Jang Y. et al., Free Rad Biol Med 2016, 95, 190).
All forms of vitamin E undergo metabolic degradation in the liver. Thus, garcinoic acid (δ-T3-carboxychromanol) is a natural tocotrienol metabolite that has preliminarily been identified as a modulator of nuclear receptors involved in β-amyloid (Aβ) metabolism and progression of Alzheimer’s disease. It holds potential for preventing Aβ oligomerization and deposition in the brain (Marinelli R et al., J Biol Chem 2020, July 2).
It has been shown that sulfated long-chain carboxychromanols were generated in cultivated cells from man and rats (Jiang Q et al., J Lipid Res 2007, 48, 1221). These studies demonstrated that sulfation occurred at long-chain carboxychromanols, the intermediate metabolites generated from β-oxidation of the phytyl chain, the sulfation rate varying between vitamin E forms.
An extensive overview of vitamin E catabolism may be consulted (Wu JH et al., Mol Aspects Med 2007, 28, 437).
While glycosylated derivatives of tocopherols have not been yet detected in plant tissues, glycosylation of tocopherols has been demonstrated in cultured suspension cells of Eucalyptus perriniana (Shimoda K et al., Phytochemistry 2007, 68, 2678). α-Tocopherol was shown to be converted mainly into a mono-glucoside derivative (α-tocopheryl 6-0-β-D-glucopyranoside) and a di-glucoside derivative (α-tocopheryl 6-0-(6-0-β-D-glucopyranosyl)-β-D-glucopyranoside or α-tocopheryl β-gentiobioside). Small amounts of α-tocopheryl 6-0-(6-0-β-L-rhamnopyranosyl)-β-D-glucopyranoside (or α-tocopheryl β-rutinoside) were also formed. δ-Tocopherol was also converted into mono- and di-glycosylated derivatives.
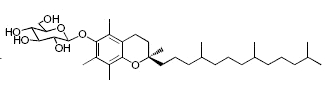
α-Tocopheryl 6-0-β-D-glucopyranoside
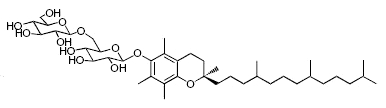
α-Tocopheryl 6-0-(6-0-β-D-glucopyranosyl)-β-D-glucopyranoside
These new di-glycosylated derivatives may act as potent anti-allergic agents as synthetically synthesized mono-glycosylated derivatives.
![]()
The oxidation of α-tocopherol to quinones and related intermediates is of considerable interest in connection with the mode of action of vitamin E. It has been realized, almost since the vitamin was discovered, that its activity is related to its antioxidant properties. Many consider that the biological role of vitamin E in animals is solely that of a physiological antioxidant.The first oxidation product of a-tocopherol, metabolically produced by trapping peroxyl radicals, is a tocopheroxyl radical. The reaction of this tocopheroxyl radical with lipid peroxides yield mainly an unstable product: 8-substituted tocopherones (α-tocopheroxides: alkyldioxytocopherones or hydroxytocopherone) which readily hydrolyze in acidic conditions to tocopherol quinone (TQ), this product being eventually reduced reversibly into tocopherolhydroquinone (THQ). Another way is currently accepted, that is the production of isomeric epoxytocopherones which further hydrolyze in acidic conditions to epoxyquinones (TQE1 et TQE2). An extensive overview of vitamin E metabolism may be consulted (Wu JH et al., Mol Aspects Med 2007, 28, 437).
|
|
|
|
|
|
|
The knowledge of the content of these compounds has been proposed as an index of oxidation of membrane lipids (Liebler DC et al., Anal Biochem 1996, 236, 27; Jain SK et al., J Am Coll Nutr 1996, 15, 44).
Similarly, oxidation products of α-tocotrienol have been described in food matrices (Busing A et al., J Agric Food Chem 2012, 60, 8302). Besides α-tocotrienolquinone, two epoxide derivatives and two formyl derivatives have been detected.
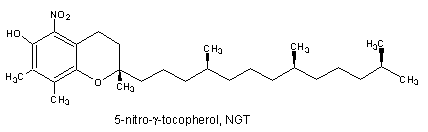
It was demonstrated that the reaction of g-tocopherol with reactive nitrogen oxide species (RNOS) such as peroxinitrite is fundamentally different to that of α-tocopherol (Christen S et al., Proc Natl Acad Sci USA 1997, 94, 3217). Thus, while RNOS oxidize α-tocopherol near quantitatively to a-tocopherol quinone, the major reaction product of γ-tocopherol with RNOS is the nitro-phenol 5-nitro-γ-tocopherol. Furthermore, It has been demonstrated that g-tocopherol is a target for nitration in vivo since the concentrations of 5-nitro-γ-tocopherol are elevated in the plasma of subjects with coronary heart disease and in carotid-artery atherosclerotic plaque (Morton LW et al., Biochem J 2002, 364, 625).
The absorption peaks for the various substituted tocols are given in the following table.
|
MW |
l max (nm) |
mol. abs. |
|
| α-tocopherol |
430.7 |
294 |
3056 |
| γ-tocopherol |
416.7 |
298 |
3867 |
| δ-tocopherol |
402.7 |
298 |
3673 |
| α-tocotrienol |
424.7 |
292 |
3865 |
| β-tocotrienol |
410.7 |
296 |
3573 |
| δ-tocotrienol |
396.7 |
292 |
3293 |
| α-tocopherol quinone |
446.7 |
262 |
19500 |
| α-tocopherol acetate |
472.7 |
285 |
2034 |
Tocopherols (except acetate and quinone) fluoresce strongly, emitting at 340 nm when excited around 295 nm.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire