
An amide bound between a fatty acid and the amine group of sphingosine or other related amino alcohols gives rise to a ceramide. Attachment of one sugar group by O-ester linkage to the primary alcohol of the ceramide yields a ceramide hexoside.
In animals the galactocerebrosides (most commonly known as cerebrosides, or GalCer) are the glycosphingolipids the most frequently found (in brain tissue). The fatty acid (with 20 to 24 carbon atoms) is normal or 2-hydroxylated (mainly in kidney and brain), the long-chain base being sphingosine or dihydrosphingosine.
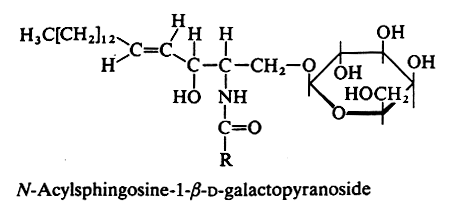
An acetylated derivative of GalCer (3-O-acetyl-sphingosine GalCer) has been characterized in rat brain myelin (Dasgupta S et al. J Lipid Res 2002, 43, 751). That is, an acetyl group is linked at the C3-OH of the sphingosine base.
Cerebrosides were the first glycosphingolipids discovered in brain tissue and named by L.W. Thudichum in 1874. He demonstrated that they are composed of a fatty acid, a long-chain base and a sugar. The hexose component was identified as galactose by Thierfelder (Z Physiol Chem 1890, 14, 209).
Due to this unique structure, cerebrosides have various biological activities and, as a result, have attracted pharmaceutical research attention. The production of corresponding α-anomers in mammals have not been described so far, but it has been proposed that at very low levels α-GalCer (a synthetic glycosphingolipid) may be present and necessary for invariant natural killer T cell homeostasis (Kain L et al., Immunity 2014, 41, 543). α-Galactosylceramide can be used to treat conditions such as atopy, cancer, infection and autoimmunity. It has been shown that oral delivery of a liposomal α-GalCer can stimulate local and systemic immune responses (Kaneko K et al., J Pharm Pharmacol 2017, 69, 1724).
Similar compounds with a α-galactosyl linkage have been described in the marine sponge Agelas mauritianus (Natori T et al., Tetrahedron Lett 1993, 34, 5591). These glycolipids, named agelasphins, are formed by a ceramide containing a C24 hydroxylated fatty acid and phytosphingosine-like base with 16 to 19 carbon atoms. All these glycolipids showed antitumor activity. KRN7000 is a synthetic analog of an agelasphin. It is a specific ligand for human and mouse natural killer T cells (NKT cells) and remains the best studied ligand of the lipid-binding MHC class I-like protein CD1d. It protects against LPS-induced shock and displays potent antitumor activity in various in vivo models. The potential of a-GalCer stimulated iNKT cells in tumor treatment is actively investigated.
Cerebrosides are concentrated in nervous tissues where they increase from about 0.02% of the dry weight in human fetal brain to adult levels of 2% in gray matter and 12% in white matter.
In Li et al.’s study (Li Q et al., Mol Nutr Food Res 2019, 63, 1800707), an Alzheimer disease rat model was obtained by intraventricular injection of Aβ1–42 and treated with cerebrosides by intragastric administration. Initially, the Morris water maze results indicated that oral administration of sea cucumber cerebrosides could significantly ameliorate the impaired cognitive function in Aβ1–42-treated rats. Moreover, sea cucumber cerebrosides improved synaptic plasticity.
Cerebrosides were also shown to be present in larval and adult forms of the tapeworm, Spirometra erinacei (Kawakami Y et al., Lipids 1995, 30, 333). The hexose consisted primarily of galactose, the sphingoid base was sphinganine (d18:0) or phytosphingosine (t18:0) and the fatty acid ranged from 16 to 30 carbon atoms, hydoxy stearic acid being also found.
Cerebrosides are largely absent in the skin stratum corneum of most vertebrates, and in some mammals their presence represents a pathology that disrupts the lipid barrier. However, cerebrosides make up approximately 15% of all lipids in birds and 5% in bats, increasing to even greater proportions in species living in arid environments (Champagne AM et al., Chem Phys Lipids 2016, 195, 47). Similarities in lipid composition between alligator and avian stratum corneum have been described, including a high percentage of cerebrosides (Tam EA et al., Comp Biochem Physiol Part A: Mol Integr Physiol 2024, 292, 111620), Authors hypothesized that selection for lipids in Archosaurian stratum corneum was driven by the unique distribution of proteins in the skin of this clade, cerebrosides serving as pre-adaptations for flight.
A glucosylceramide (glucocerebroside) was first isolated in 1940 from the spleen of a man with Gaucher’s disease (Halliday N et al., J Biol Chem 1940, 132, 171). Later, it was isolated in several extra-neural organs. In erythrocytes glucose is detected. It was also shown to be present consistently in multiple-drug resistant cell lines where it may hold significance for the early identification of drug-resistant tumors (Lucci A et al., Anticancer Res 1998, 18, 475).
A phylogenetic dichotomy of nerve glycosphingolipid has been established in studying nervous tissues from a wide variety of animals (Okamura N et al., Proc Natl Acad Sci 1985, 82, 6779). It appears that nerves of protostome animals contain only glucocerebrosides while galactocerebrosides are mainly present in deuterostome animals. This correlation suggests that an evolutionary trend which corresponds with the genesis of highly structured myelin around axons in deuterostomes.
Cerebroside (monohexoside) storage in excessive amounts in the brain leads to Gaucher’s disease characterized by spleen and liver enlargement and mental retardation.
Since 1988, it has been realized that cerebrosides self-aggregate in cellular membranes to form a separate phase that is less fluid than the bulk phospholipids based on diacylglycerol. Sphingolipid-based microdomains or “rafts” were originally proposed to sort membrane proteins along the cellular pathways of membrane transport (Simons K et al., Biochemistry 1988, 27, 6197). Presently, most excitement focuses on their organizing functions in signal transduction (Brown DA et al., J Biol Chem 2000, 275, 17221). The importance of rafts and translocation of sphingolipids have been reviewed (Van Meer G et al., J Biol Chem 2002, 277, 25855).
Glycosphingolipids like galactosylceramides are used as cellular binding sites for a wide variety of pathogens, including viruses, bacteria, fungi and parasites (see web site).
Original glucocerebrosides (renierosides) have been studied in a marine sponge, Reniera sp. These forms were shown to have amide-linked long-chain hydroxylated fatty acid moieties (C25, C26 or C28) and a sphingoid base with three double bonds (Mansoor T et al., J Nat Prod 2007, 70, 1481).
In plants, the existence of glycosphingolipids was documented only in 1954 (Carter HE et al., J Biol Chem 1954, 206, 613). In plants, glucose is found instead of galactose, the fatty acid is most frequently hydroxylated (2-hydroxy with 16 or 18 carbon atoms) and the long-chain base is phytosphingosine or dehydrophytosphingosine. Monoglucosecerebrosides containing 4,8-sphingadienine have been described in lipid extracts from soybean and nuts of almond (Prunus amygdalus)(Shibuya H et al., Chem Pharm Bull 1990, 38, 2933; Sang S et al., J Agric Food Chem 2002, 50, 4709). These compounds have been reported to exhibit significant biological activities, such as anti-ulcerogenic, ionophoretic and anti-hepatotoxic activities. A glucosphingolipid based on dehydrophytosphingosine and hydroxylated fatty acid of various length has been described in an Euphorbiaceae (Euphorbia sororia) (Zhang WK et al., Chem Phys Lipids 2007, 148, 77). This compound has neuritogenic activity as other parent compounds isolated from mushroom (Qi JH et al., Tetrahedron 2001, 56, 5835).
Glucosylceramide is a major sphingolipid in plants such as pineapple, wheat, rice, corn, soybean, and konjac (Uchiyama T et al., J Health Sci 2008, 54, 559). Orally ingested glucosylceramide gets hydrolyzed to become a sphingoid base that is absorbed in the intestine and it enhances the synthesis of ceramide. In Japan, glucosylceramide extracted and purified from pineapple fruits has been reported to be safe for consumption and has been approved as food (Yoshino, S et al., Jpn Pharmacol Ther 2016, 44, 255). Investigations have shown that oral intake of glucosylceramide may improve the moisture level and xerostomia symptoms in skin (Murakami M et al., Nutrients 2019, 11:2020).
Phyto-glucosylceramides have been shown to be important in specialized cells during cotton fiber generation (Chen O et al., Biomolecules 2021, 1352). With the development of fiber cells, phytosphingosine-1-phosphate (t-S1P) and phyto-ceramides changed greatly. Thus, the sphingolipid molecular species Ceramide d18:1/26:1, phytoCer t18:1/26:0, phytoCer t18:0/26:0, phytoCer t18:1/h20:0, phytoCer t18:1/h26:0, phytoCer t18:0/h26:0 were significantly enriched in fiber cells
A monoglucosecerebroside (pinelloside) with strong antimicrobial properties (against Gram-positive and -negative bacteria and against fungi) was described in the tuber of Pinella ternata (Araceae), one component of decoctions used in traditional Chinese medicine (Chen JH et al., Phytochemistry 2003, 64, 903). Its structure was shown to include a glucose moiety and the unusual 4,11-sphingadienine linked to a 2-hydroxy-palmitic acid. Fungal glucosylceramides can be considered non only as a structural component of cell membranes but participate also in recognition by the immune system, regulation of virulence and in cellular signaling (Nimrichter L et al., Lipid Insights 2008, 2, 61).
A glycosylcerebroside with a tri-hydroxylated C18 sphingosine analogue linked to a C17 hydroxylated fatty acid has been described as a potential biomarker for viral infection of planktonic coccolithophore populations (Vardi A et al., Science 2009, 326, 861). It was shown that it was able to induce biochemical hallmarks of programmed cell death in uninfected host, Emiliana huxleyi.
Several glucocerebrosides have been isolated from the aerial parts of Orostachys japonicus (Crassulaceae) (Zhang H et al., Food Chem 2012, 131, 1097). The most active molecule in inhibiting fatty acid synthase has the fatty acid and sphingosine moieties which were determined as 2-hydroxyeicosa-6,9-dienoic acid and 2-amino-1,3,4-trihydroxytetracosa-6,10-diene, respectively. Its cytotoxic activity may provide a scientific basis for the folk remedy using the plant to treat cancer in China and Japon.
Several glucosphingolipids have been described in wheat bran, some of them having interesting cytotoxic effects against colon cancer cells (Zhu Y et al., J Agric Food Chem 2013, 61, 866). These sphingolipids may be implicated in colon cancer prevention as a component of this food frequently considered as bioactive.
It appears progressively that plant sphingolipids are composed of structurally diverse molecules that are important as membrane components and bioactive molecules. An appreciation of the relationship between structural diversity and functional significance of plant sphingolipids is emerging through characterization of Arabidopsis mutants coupled with a lipidomics approach (Markham JE et al., Curr Opin Plant Biol 2013, 16, 350).
A new cerebroside, namely 1-O-β-D-glucopyranosyl-(2R,6E,9R,12E)-2-[(2′R)-2′-hydroxypentacosaneoyl-amino]-6,12-heptadecene-1,9-diol has been isolated from the roots of Gynura procumbens, a member of the family Compositae distributed throughout China, Malaysia,Thailand, Indonesia, Korea, and the Philippines (Hu JW et al., Chem Nat Comp 2019, 55, 1053). This plant is used as a folk medicine for the treatment of eruptive fevers, rash, kidney disease, migraines, constipation, hypertension, and diabetes mellitus.
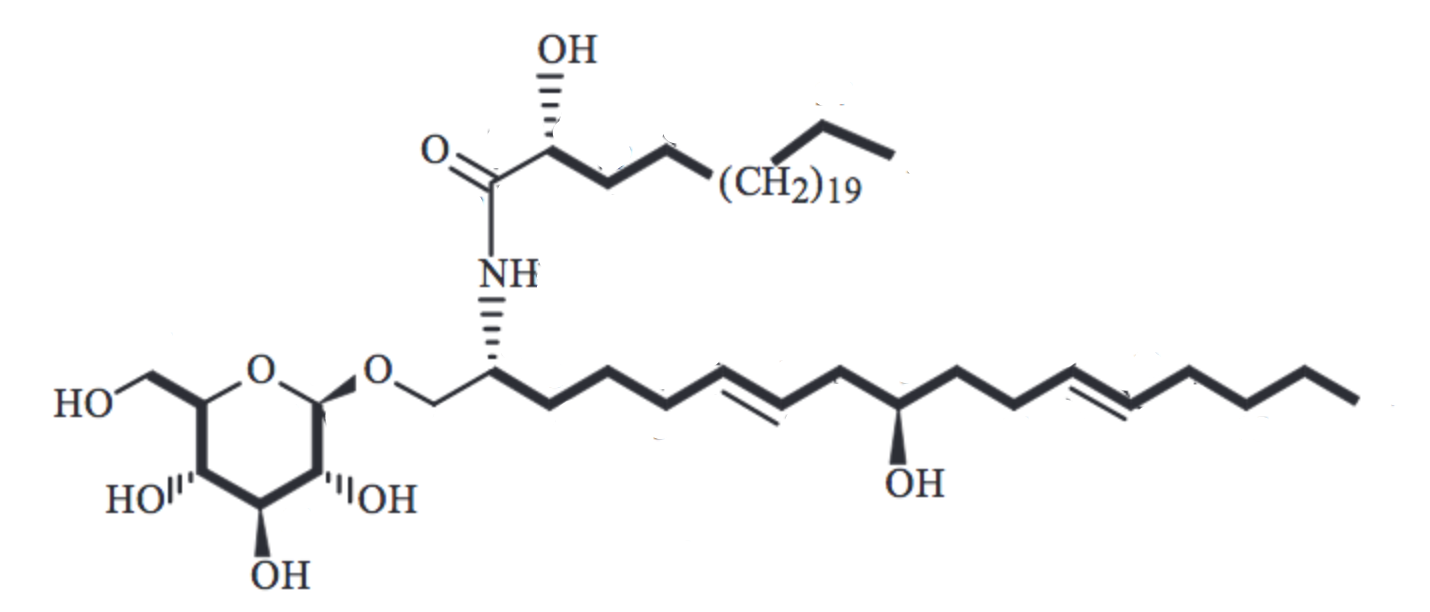
New glycolipid from Gynura procumbens
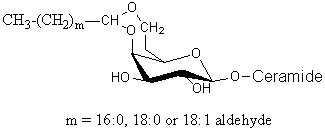
Glycosphingolipids having a long-chain cyclic acetal have been isolated from human brain and named plasmalocerebroside (Levery SB et al., Biochemistry 1992, 31, 5335). They were found as spots having much higher mobility than unmodified cerebrosides on thin-layer chromatography. These components were found to be fatty aldehyde conjugates of cerebroside, characterized by the formation of either 3,4 or 4,6 cyclic acetal linkages to the b-galactopyranosyl residue. This acid-labile structure is analogous to plasmalopsychosine. The yield of this compound amounted to 0.3 mg per Kg (wet weight) of brain tissue (4000 times lower than the concentration of galactocerebroside). Only the galactocerebroside 4,6-O-cyclic fatty acetal is shown below.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire