
We include in that type all glycoglycerolipids containing at least one phosphate group whatever its position, either attached to one sugar or to one glycerol.
If we except phosphoinositides which contain one mole of inositol and could be classified into this group but are rather considered as “phospholipids”, the glycophospholipids have been mainly described in bacteria. Some complex structures, the glycosyl phosphatidylinositol anchors, are found in all living creatures.
The simplest form of glycophospholipids are based on the simplest phospholipid, phosphatidic acid, linked to a glycosy group. The simplest of these compounds is glucosylated phosphatidic acid which was found in the red blood cells of the human umbilical cord (Nagatsuka Y et al., FEBS Lett 2001, 497, 141).
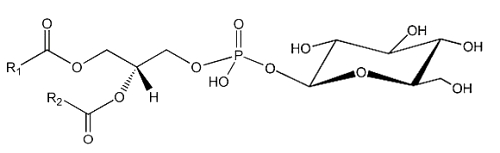
This phosphatidylglucoside was also identified in the rat brain, human neutrophils and several human epithelium cells. It could be also a new marker for lipid rafts (Nagatsuka Y et al., FEBS Lett 2001, 497, 141). Curiously, this glycolipid is composed exclusively of a single pair of saturated fatty acids (18:0 and 20:0). Its function remains unknown.
It is well known that sugars and carbonyl compounds interact with amino acids or proteins in a sequence of reactions known as the Maillard reaction. Similarly, it has been shown that phosphatidylethanolamine also reacts with glucose leading, through an unstable Schiff base, to a phosphatidylethanolamine-linked Amadori product (Bucala R et al., Proc Natl Acad Sci USA 1993, 90, 6434).
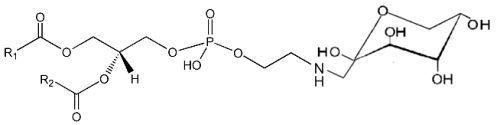
These compounds were detected in the rat liver and their concentration was increased in diabetic rats (Pamplona R et al., Life Sci 1995, 57, 873). Later, their correct structure was described in human red blood cells (Lertsiri S et al., Biosci Biotechnol Biochem 1998, 62, 893). Intensive researches have shown that Amadori-lipid products accelerate membrane lipid peroxidation in generating oxidative stress which alters cell integrity and survival (Oak JH et al., FEBS Lett 2000, 481, 26). Amadori-PE concentrations have been determined to be higher in plasma and red blood cells of diabetic patients when compared to healthy subjects (Shoji N et al., J Lipid Res 2010, 51, 2445).
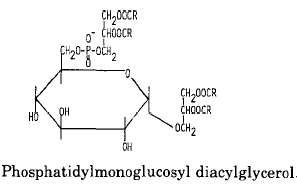
An example of glycophospholipids found in many Gram-positive bacteria is phosphatidyl monoglucosyl diacylglycerol whose structure and metabolism were elucidated in Pseudomonas diminuta (Shaw JM et al., J Biol Chem 1977, 252, 4395).
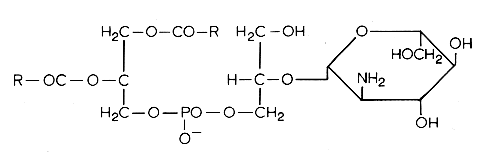
Another example of these lipids is a phosphatidyl glucosaminyl glycerol found in Bacillus megaterium
Another phosphoglycolipid has been isolated from several species of the halophilic Gram-negative bacteria Halomonas (Giordano A et al., J Lipid Res 2007, 48, 1825). The structure was established to be a phosphatidic acid linked to a glycerol residue (phosphatidylglycerol moiety) which is alkylated by a glucose group. The most abundant acyl chains linked to the phosphatidylglycerol moiety are palmitic and oleic acids and C16:0, C19:cyclopropane.
A derivative of N-acetylglucosaminyl diacylglycerol in which a phosphoethanolamine unit is attached to the 6′-position of the sugar has been isolated from Clostridium tetani, the causative agent of tetanus (Johnston NC et al., J Lipid Res 2010, 51, 1953).
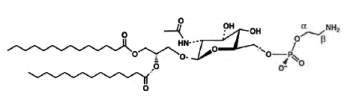
N-Acetylglucosaminyl-phosphoethanolamine diacylglycerol
Another example is phosphatidylinositol polymannoside found in Mycobacteria
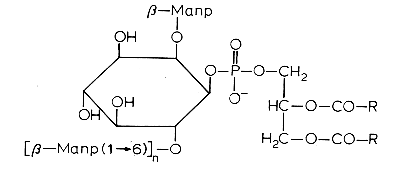
in position 2′ of the inositol there is a mannopyranoside group and in position 6′ there may be one sugar (dimannoside), three (tetramannoside), four (pentamannoside), or five (hexamannoside) sugar groups.
It was found that phosphatidylinositol mannosides from Mycobacterium tuberculosis exert potent anti-inflammatory effects in a lung model in vitro and in vivo via an inhibition of Toll-like receptor and inhibition of the production of NO, cytokines and chemokines (Doz E et al., J Biol Chem 2009, 284, 23187). These effects may be a strategy developed by mycobacteria for repressing the host innate immunity. Analogues are in development for clinical essays.
The presence of the mannophosphoinositides is a striking feature of the phospholipid composition of the Actinomycetes and of some bacteria. Monomannosides have been reported in some Streptomyces, Mycobacterium species and in Propionibacteria. Two types of dimannosides, differing in their fatty acid were identified in Streptomyces griseus. They have at least one additional fatty acid attached to a mannose residue (tri- and tetra-acylmannoside). The most complex polymannosides were identified in Mycobacteria, sometimes with several fatty acids acylating the mannose chain.
Acylated phosphatidylinositol dimannosides were also described in Corynebacterium urealyticum, a C16:0 or C18:1(R3) acylating one of the two mannose moieties (Yagüe G et al., Microbiology 2003, 149, 1675).
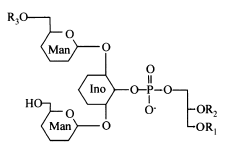
It has been shown than phosphatidylinositol dimannoside was the “anchoring domain” of the lipopolysaccharides of mycobacteria, lipoarabinomannan and lipomannan, of key importance in host-pathogen interaction (Hunter SW et al., J Biol Chem 1990, 265, 9272). This structure was the first demonstration of a prokaryotic version of the phosphatidylinositolglycans which are well known in anchoring cell-surface proteins (Ferguson MAJ et al., Annu Rev Biochem 1988, 57, 285). The complete structural features of lipoarabinomannan from Mycobacterium bovis, largely used around the world as vaccine against tuberculosis, have been described (Venisse A et al., J Biol Chem 1993, 268, 12401).
A review of the distribution and composition of these glycophospholipids and of other Actinomycetes lipids may be consulted with interest (Verma JN et al., Adv Lipid Res 1983, 20, 257).
Lipophosphoglycan is the predominant glycolipid present at the surface of parasitic protozoa such as Leishmania, Entamoaba and Crithidia (review in: Guha-Niyogi A et al., Glycobiology 2001, 11, 45). It is composed of four domains, (1) an alkyl lyso phosphatidylinositol anchor, (2) a glycan core, (3) Gal-Man-PO4 backbone repeated units, and (4) an oligosaccharide cap structure (Descoteaux A et al., Biochim Biophys Acta 1999, 1455, 341). Among species, the lipid anchor, the glycan core and the Gal-Man-PO4backbone are completely preserved while variations are found in the carbohydrate chain and in the cap structure (Mc Conville MAJ et al., Biochem J 1995, 310, 807). Lipophosphoglycan has been implicated in a number of functions within the mammalian host.
Glycosylated cardiolipin was first detected in several strains of Streptococcus group B and identified as glucopyranosyl cardiolipin (Fisher W, Biochim Biophys Acta 1977, 487, 74).
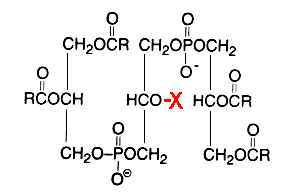
X = glucosyl residue
This derivative amounts to about 18% of the lipid phosphorus in Streptococcus but is only a minor component in Vagococcus fluviatilis (Fisher W et al., J Bacteriol 1998, 180, 2950). This glycolipid is acylated mainly by palmitic acid (about 33%) and oleic acid (about 25%) (Fisher W, Biochim Biophys Acta 1977, 487, 89). This uncommon glycolipid was also found (4 mol% of total phospholipids) in a thermophilic bacteria Geobacillus steathermophilus where its fatty acid pattern exhibits predominantly iso-C15:0 and anteiso-C17:0 (Schäffer C et al., J Bacteriol 2002, 184, 6709). It was hypothesized that this compound could be involved in the regulation of the membrane lipid composition of G. stearothermophilus to compensate for the destabilizing effect of high temperatures on the membrane organization.
Several cardiolipin analogues of extremely halophilic prokaryotes belonging to the Archaea domain (Halobacterium salinarum) have been described (Corcelli A, Biochim Biophys Acta 2009, 1788, 2101).
A unique glycophospholipid present at a level of 33% of the total lipids has been identified in a Gram-positive bacteria Deinococcus radiodurans (Anderson R, Biochim Biophys Acta 1983, 753, 266).
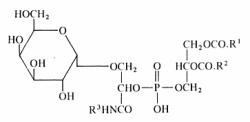
This lipid was described as the 3-(galactopyranosyl)-2-(3-phosphatidyl) glyceroyl derivative of a fatty alkylamine. The alkylamines (R3) are mainly straight-chain compounds, saturated (C15 to C17) or monoenoic (C16 to C18), with isomeric 17:1 bases preponderant.
Mycoplasma fermentans was shown to produce a phosphocholine-containing glycoglycerolipid which has the phosphocholine group attached to the C6 of glucose (Matsuda K et al., J Biol Chem 1994, 269, 33123).
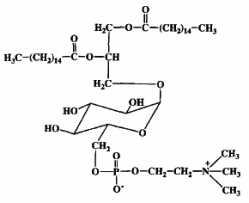
It has been hypothesized that this lipid might be involved in the pathogenesis of some diseases, as rheumatoid arthritis, through triggering of inflammation or cell death caused by their phosphocholine residue.
The chemical structure of another choline-containing phosphoglycolipids with two phosphate residues was described in M. fermentans. The major phosphoglycolipid (MfGL-II), which accounts for about 30% of the total polar lipid fraction, was identified as 6′-O-[3″-phosphocholine-2″-amino-1″-phospho-1″,3″ -propanediol]-alpha-D-glucopyranosyl-(1′-3)-1,2-diacyl-glycerol (Zahringer et al., J Biol Chem 1997, 272, 26262).
In the Gram-positive bacteria Lactococcus and Streptococcus, several complex glycolipids deriving from the kojibiosyl diacylglycerol structure (glucopyranosyl-1->2-glucopyranoside-diacylglycerol) were identified (O’Leary WM et al., in “Microbial lipids” vol 1, Ratledge C et al. eds, Acad Press, 1988).
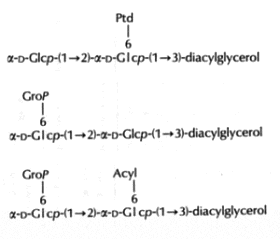
(Ptd: 3-phosphatidyl, Grop: glycero-1-phospho, Acyl: fatty acyl group)
Typically, these structures are derived by substitution at one or both of the 6-positions on glucose by a 3-phosphatidyl group, a glycerol-1-phospho group, or a fatty acyl group. In more complex cases, additional glycerophospho residues (and glycosyl and alanyl substituents) are incorporated (Fischer W in “Chemistry and biological activities of bacterial surface amphiphiles”, Shockman GD et al., eds, Acad. Press 1981).
In membranes of Gram-positive bacteria (ex. Staphylococcus) more complex phosphorylated compounds can be found: lipoteichoic acids. They consist of polymer of glycerol-1-phosphate linked to a glycosyl diglyceride or a phosphatidyl glycosyl diglyceride. Some other substitutions on the glycerophosphate units with glycosyl or alanyl groups are also described (Lambert et al., Biochim Biophys Acta 1977, 472, 1).
The lipoteichoic acid found in Bacillus subtilis is illustrated below :
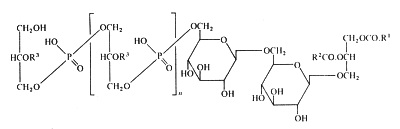
R1 et R2 are fatty acyl groups and R3 is mainly H, alanyl or acetylglucosaminyl
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire