
Also referred historically to cardiolipin, this curious lipid is found almost exclusively in mitochondria and in bacteria. It can account for as much as 20% of mitochondrial lipids. Cardiolopin was first discovered in beef heart tissue (Pangborn MC, J Biol Chem 1942, 143, 247) but, later, it was recognized to be not specific to the heart.
This phospholipid was discovered in 1906 by Wasserman through its antigenic properties in attempting to isolate the substance that confers on alcoholic extracts of beef heart the property of reacting with sera from cases of syphilis (Wassermann test) (Wassermann A et al., Dtsch Med Wochenschr 1906, 32, 745). A popular serological test, similar to the Wassermann reaction for detection of syphilis, used a mixture of cardiolipin, phosphatidylcholine and cholesterol (for details on antiphosphospholipid antibodies see the review of McIntyre JA et al. (Prog Lipid Res 2003, 42, 176 ). Since 1911, it is known that the antigenic activity is associated with the acetone-insoluble portion of the heart extract. Mrs Mary C. Pangborn was the first to “isolate and purify a serologically active phospholipid from beef heart“. She proposed to designate this compound “cardiolipin” (heart lipid). In her first report (Pangborn M., Proc Soc Exp Biol Med 1941, 48, 4 84) she claimed that :
| “A new phospholipid from beef heart has been isolated and purified. On hydrolysis it yields fatty acids and a phosphorylated polysaccharide. The name cardiolipin is suggested for this substance, which is essential for the reactivity of beef heart antigens in the serologic test for syphilis“ |
Later, she improved and simplified the preparation methodology (Pangborn MC, J Biol Chem 1944, 153, 343). She reported that the previous observation of a carbohydrate component in the molecule was an error “apparently due to persistent traces of carbohydrate impurities“. At that time, the purification was entirely based on solvent properties (methanol, ethanol, acetone, ether, benzene, ethyl acetate, chloroform, water) and barium salt insolubility, but no information on the composition of this lipid was given.
Evidence that cardiolipin is composed of three glycerol, two phosphoric acid and four fatty acid residues was brought in 1958 by MacFarlane MG et al. (Biochem J 1958, 70, 409, Nature 1958, 182, 946). One year later, she proposed the exact position of the fatty acids (R in the figure below) in the cardiolipin molecule (Nature 1959, 180, 1808). This discovery was possible thanks to the report of a diacylglycerol liberation from a phospholipid fraction in hot acetic acid (Coulon-Morelec MJ et al., C R Acad Sci, Paris 1958, 246, 1936). The mechanism of this reaction was then studied and the liberation of the diacylglycerol was shown to be linked to the presence of a free hydroxyl adjacent to the phosphoric acid, condition present in cardiolipin and phosphatidylinositol (Coulon-Morelec MJ et al., Bull Soc Chim Biol 1960, 42, 867).
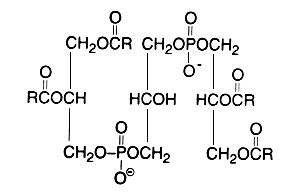
This phospholipid can be defined as 1,3-bis(sn-3-phosphatidyl)-sn-glycerol. Curiously, mammalian cardiolipin contains up to 90 mol% of one fatty acid, linoleic acid (Hoch FL, Biochim Biophys Acta 1992, 1113, 71).
The most abundant cardiolipin species from various organisms and tissues (human heart, human lymphoblasts, rat liver, Drosophila, sea urchin sperm, yeast, vegetal cells) contained only one or two types of fatty acids, which generated a high degree of structural uniformity (Schlame M et al., Chem Phys Lipids 2005, 138, 38). In contrast, it was demonstrated the presence of changes in the cardiolipin molecular species profile in the mammalian brain during the perinatal period. These changes are correlated to the massive alterations in neuronal remodeling and apoptosis that occur after birth (Cheng H et al., Biochemistry 2008, 47, 5869). Over 100 molecular species have been identified. The embryonic cardiolipin profile was notable for the presence of abundant amounts of relatively short fatty chains. After birth, a more complex range of molecular species, rich in arachidonic and docosahexaenoic acids, was observed. Similarly, cardiolipin compositional abnormalities involving abundance of immature molecular species (shorter chain saturated or monounsaturated fatty acids) have been observed in brain tumor mitochondria, results which support the Warburg theory of cancer (Kiebish MA et al., J Lipid Res 2008, 49, 2545).
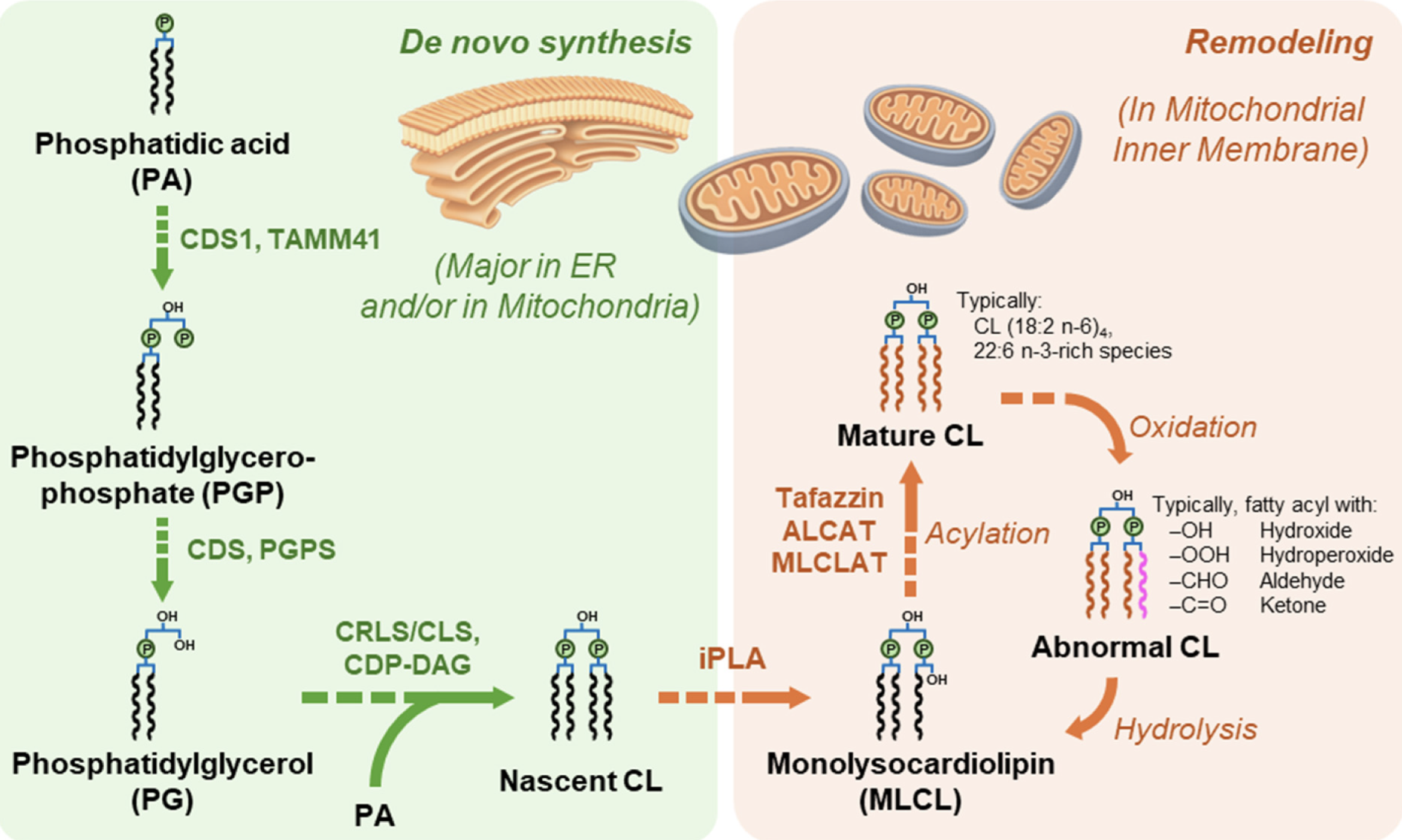
Main pathways of cardiolipin biosynthesis and remodeling
(from Dong C et al., Biomolecules 2026, 16, 71)
In some marine bivalves (Pecten maximus, Crassostrea gigas and Mytilus edulis), cardiolipin molecules are found predominently with four docosahexaenoyl chains (22:6 n-3) and are presumed to reflect a specific adaptation to environmental conditions (Kraffe E et al. Lipids 2002, 37, 507). Cardiolipin from a Manila clam, Ruditapes philippinarum, was shown to contain EPA and DHA in approximately equal proportions and contributing together up to 73% of the total fatty acids of that phospholipid (Kraffe E et al., Lipids 2005, 40, 619). A survey of the cardiolipin fatty acid composition among thirty-five species of marine mollusks revealed a control and a conservation of that composition in species of the same phylogenetic group (Kraffe E et al., Lipids 2008, 43, 961).
Although some microorganisms lack cardiolipin, it comprises 2-25% of lipid phosphorus in the majority of bacteria. Several species contain up to 38% (Bacillus subtilis), 59% (Streptococcus) and even 79% (Staphylococcus aureus). A diphytanylglycerol ether analogue of cardiolipin has been isolated from the purple membranes of Halobacterium salinarum (Corcelli A et al., Biochemistry 2000, 39, 3318). An important fraction of cardiolipin plasmalogen (with one or two alk-1′-enyl chains) has been reported in lipid extracts of Clostridium novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193).
A lipidomic approach of two haloalkaliphilic archaeal microorganisms of the genus Natronococcus has yielded detailed information on novel cardiolipins based on 2-O-sesterpanyl-3-O-phytanyl- sn-glycerol (C25, C20) diether lipid cores (Angelini R et al., Biochim Biophys Acta 2012, 1818, 1365). The presence of two C25 and two C20 lipid chains (Zip structure) in this phospholipid of haloalkaliphilic archaea could make more stable the membrane, conferring the ability to better withstand high pH gradients in the presence of very high salt concentrations (De Rosa M. et al., J Gen Microbiol 1982, 128, 343).
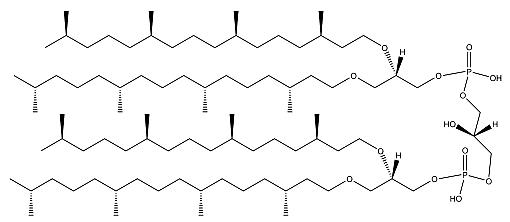
Ether cardiolipin from Natronococcus
Cardiolipin has been extensively studied since it was found to be associated to cytochrome oxidase in the electron transport system located in the mitochondrial cristae membranes, in chloroplast thylakoid membranes, and in bacterial membranes (review in Robinson NC, J Bioenerg Biomembr 1993, 25, 153). Cardiolipin is emerging as an important factor in the regulation of mitochondrial bioenergetics in that it interacts with several vital inner membrane proteins, including anion carriers and respiratory chain complexes. Although its exact function remains to be defined, it seems to be a potential factor in several pathologies, such as thyroid disease (Paradies GFM et al., Arch Biochem Biophys 1993, 307, 91), oxidative stress (Iwase HT et al., Biochem Biophys Res Comm 1996, 222, 83), and aging (Paradies GFM et al., FEBS Lett 1997, 406, 136). It has been demonstrated that mitochondrial dysfunction associated with cardiac ischemia-reperfusion is associated with cardiolipin oxidation. Melatonin was able to protect against such dysfunction and oxidative alteration of cardiolipin (Petrosillo G et al., FASEB J 2006, 20, 269).
Disruptions in cardiolipin metabolism are increasingly implicated in the pathogenesis of various central nervous system (CNS) disorders, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, epilepsy, and traumatic brain injury (Falabella M et al., Trends Endocrinol. Metab. 2021, 32, 224).
Accumulating evidence suggests that this unique lipid also has active roles in several of the mitochondria-dependant steps of apoptosis (cytochrome c dissociation from the mitochondrial inner membrane, permeabilization of the mitochondrial outer membrane) (Gonzalvez F et al., Apoptosis 2007, 12, 877). The complex role of cardiolipin in human health and disease has been reviewed (Claypool SM et al., Tr Biochem Sci 2012, 37, 32). A review of the relationship between cardiolipin and lipid intakes and pathophysiology in human may be consulted (Feillet-Coudray C et al., Cah Nutr Diet 2015, 50, 331).
Although it has long been known that cardiolipin plays an important role in mitochondrial bioenergetics, recent evidence in the yeast model indicates that it is also essential for intermediary metabolism. Findings obtained with a cardiolipin-deficient cell line indicate that this phospholipid is required for optimal pyruvate dehydrogenase activation, generation of acetyl-CoA, and TCA cycle function, findings that link the key mitochondrial lipid cardiolipid to TCA cycle function and energy metabolism (Li Y. et al., J Biol Chem July 26, 2019).
The fluorescent dye 10-N-nonyl acridine orange is extensively used for location and quantitative assays of cardiolipin in living cells on the assumption of its high specificity for cardiolipin (Garcia Fernandez MI et al., Anal Biochem 2004, 328, 174; Kaewsuya P et al., Anal Bioanal Chem 2007, 387, 2775).
It was shown that a lyso precursor of cardiolipin (monolysocardiolipin) could be used as a specific marker for the Barth Syndrome (an X-linked recessive disorder) when measured in patient fibroblasts (van Werkhoven MA et al., J Lipid Res 2006, 47, 2346). The analysis of monolysocardiolipin by HPLC-MS was shown to be a powerful tool to diagnose patients with clinical signs of Barth syndrome among those who suffer from cardiomyopathy with unknown etiology (Houtkooper RH et al., Anal Biochem 2009, 387, 230). The differential diagnosis of these patients includes any male child who has neutropenia, dilated cardiomyopathy with endocardial fibro-elastosis, and abnormal mitochondria in the heart. These patients may also exhibit idiopathic cardiomyopathy with growth retardation as well as 3-methylglutaconic aciduria.
This increase in monolysocardiolipin content is accompanied by a decrease in tetra-linoleyl species of cardiolipin (review in: Hauff KD et al., Prog Lipid Res 2006, 45, 91). These defects could be the result of mutations in the gene TAZ which control the production of proteins known as tafazzins.
A large review on the biosynthesis and functional role of cardiolipin was proposed by Schlame M et al. (Prog Lipid Res 2000, 39, 257). Its role in mitochondrial metabolism has been reviewed in 2008 (Houtkooper RH et al., Cell Mol Life Sci 2008, 65, 2493; Klingenberg M, Biochim Biophys Acta 2009, 1788, 2048). Its organization, its role in ATP synthesis and in Barth’s syndrome has been released (Haines TH, Biochim Biophys Acta 2009, 1788, 1997).
The study of cardiolipin profiles in different mouse tissues across different age groups has revealed that molecular species in the brain differ significantly from those in peripheral organs, with a tendency towards longer-chain variants. That study highlights a dynamic adaptation of cardiolipin profiles in response to fatty acids availability and aging and emphasizes its functional importance for mitochondrial function (Weiss PW et al., Biochim Biophys Acta Mol Cell Biol Lipids 2025, 1870, 159687).
As phosphatidylglycerol, it must be extracted from tissue homogenates using acidic solvents and some precautions are needed to prevent its loss in aqueous solutions. A review summarizes recent advances in the analytical techniques employed for cardiolipin analysis (Dong C et al., Biomolecules 2026, 16, 71).
Cardiolipin has been identified in quite all mammalian tissues (not found in erythrocytes, skin…) where it constitutes 2-10% of total phospholipids. It occurs also in invertebrates, plants, algae, yeast and bacteria. The presence of cardiolipin in prokaryotes was used to support the hypothesis of the bacterial origin of mitochondria in eukaryotes.
Alanylcardiolipin was isolated and characterized from a streptococci, Vagococcus fluviatilis (Fisher W et al., J Bacteriol 1998, 180, 2093). This phospholipid was shown to contribute up to 38% of the lipid phosphorus. Similarly, a lysyl derivative of cardiolipin has been described first in several species of Listeria, a gram-positive bacteria (Peter-Katalinic J et al., J Lipid Res 1998, 39, 2286) where it could be valuable as chemotaxonomic marker. In that phospholipid, the hydroxyl group of the middle glycerol moiety is esterified with a lysyl residue.
A glucosylated cardiolipin was described in several strains of Streptococcus (Fisher W, Biochim Biophys Acta 1977, 487, 74).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire