
Analogous structures to those described for choline phospholipids exist for the ethanolamine phospholipids. The pattern of fatty acid distribution generally shows a higher degree of unsaturation than that found in choline phospholipids. In contrast, the phosphatidylethanolamines of amoebae (Acanthamoeba castellanii) are significantly different in comparison to other cells: they contain very long fatty acyl chains. Thus, the presence of 28- and 30-carbon fatty acyl residues was confirmed, the majority of these fatty acyl residues were identified as 28∶1/20∶2, 30∶2/18∶1, 28∶0/20∶2, 30∶2/20∶4 and 30∶3/20∶3 (Palusinska-Szysz M et al., PLoS One 2014, 9:e101243). These fatty acids could serve as specific biomarkers for the presence of these microorganisms. A diacyl phosphatidylethanolamine with two branched chains (a15:0-i15:0 PE) was shown to be present in great amounts in cell membranes of Akkermansia municiphila, a common gut bacteria which has been robustly associated with positive systemic effects on host metabolism, favourable outcomes to checkpoint blockade in cancer immunotherapy and homeostatic immunity. That phospholipid was shown to efficiently recapitulate the immunomodulatory activity of the bacteria in cell-based assays (Bae M et al., Nature 2022, 608, 168).
The diacyl derivative (1,2-diacyl-sn-glycero-3-phosphorylethanolamine, phosphatidylethanolamine) was originally named “cephalin”. This phospholipid is a zwitterion over the pH range of 2-7 and is in the anionic form in the range 7-10.
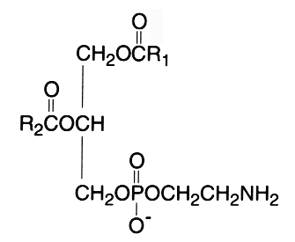
In mammalian and plant tissues this component usually occurs in lesser amounts than phosphatidylcholine. In bacteria, it is the principal phospholipid present. Thus, dimyristoyl phosphatidylethanolamine with a myristic acid (C14:0) as the saturated fatty acid side chain is one of the most abundant lipid components in Helicobacter pylori membranes, in contrast to other Gram-negative bacteria.
Several experiments have provided data related with a role of phosphatidylethanolamine in the formation of diacylglycerol as second messenger. It appears that this mechanism plays a role in the transformation and differentiation of cells (McNulty S et al., Neurosci Lett 1991, 139, 183; Lang D et al., J Neurochem 1995, 65, 810; Momchilova A et al., Int J Biochem cell Biol 1999, 31, 311). The importance of that messenger production in cell signaling remains to be reinvestigated.
The formation and the multiple functions of phosphatidylethanolamine in mammalian cells have been reviewed (Vance JE et al., Biochim Biophys Acta 2013, 1831, 543).
Activated platelets and monocytes were shown to generate phosphatidylethanolamine with 15-HETE or 12-HETE acylated at the sn-2 position of glycerol (Maskrey BH et al., J Biol Chem 2007, 282, 20151). It was determined that the formation of these phosphatidylethanolamine species in immune cells could contribute to lipoxygenase signaling in inflammation. Monocytes and macrophages generate phosphatidylethanolamine with a keto acid, 15-ketoeicosatetraenoic acid, which is a bioactive mediator activating peroxysome proliferator-activated receptor (Hammond VJ et al., J Biol Chem 2012, 287, 41651). Besides phosphatidylserine, five phosphatidylethanolamine species are externalized during the activation of platelets by thrombin or collagen (Clark SR et al., PNAS 2013, 110, 5875).
Phosphono derivatives : After the discovery of 2-aminoethylphosphonic acid in lipid extracts of the sea anemone (Kittredge JS et al., Biochemistry 1962, 1, 624), unusual phosphatidylethanolamine analogues containing a carbon-phosphorus bond instead of the classical carbon-oxygen-phosphorus bond have been described in marine invertebrates and protozoa (Tetrahymena pyriformis). They were first reported in 1966 in Tetrahymena as glycerophosphonolipids (Liang CR et al., Biochim Biophys Acta 1966, 125, 548) but, later, they were identified in that species as derivative of chimyl alcohol (Thomson GA, Biochemistry 1967, 6, 2015) : an alcohol is linked with an ether bond in sn-1 of the glycerol molecule. They were also identified in fish, some mammals, egg yolk, insects, several invertebrates and even in some plants (Mukhamedova KS et al., Chem Nat Compounds 2000, 36, 329). Thus, egg yolk contains about 1% of phosphonolipids, mainly all as phosphatidylethanolamine. Phosphonolipids have been identified in corals, in jellyfishes and in actiniae (Imbs AB et al., Mar Drugs 2023, 21, 539).
These phosphonolipids, often termed phosphonylethanolamine, are extremely resistant to acid or enzymatic hydrolysis. They were proposed as a possible artificial pulmonary surfactant.
The alk-1′-enyl, acyl derivative (ethanolamine plasmalogen) occurs widely in nature, mainly present in high concentration in the white matter (myelin) of the nervous system, in the heart and the kidney. It is practically absent in fungi, plants and bacteria (except in obligate anaerobes). The use of advanced techniques has permitted the identification of not only plasmalogen classes in bacteria but also their positional isomers and often also individual molecular species (Rezanka T et al., Folia Microbiol 2012, 57, 463).
.
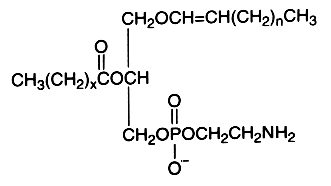
The fatty acid acylated at the 2-position of glycerol is most frequently polyunsaturated (arachidonic or docosahexaenoic acid). Ethanolamine plasmalogens constitute more than 80 mol% of the ethanolamine phospholipid pool in non-neuronal brain membranes and more than 60 mol% in neurons and synaptosomes (Han X et al., J Neurochem 2001, 77, 1168). Those found in white matter contain predominantly 18:1, 20:1, and 22:4 fatty acids at the sn-2 position, whereas in gray matter, 22:6, 20:4, and 22:4 are found in the highest concentrations (Horrocks LA et al., In Phospholipids. J. N. Hawthorne and G. B. Ansell, editors. Elsevier, Amsterdam, 1982, 51–93).
Ethanolamine plasmalogens are the predominant form in numerous organs except in heart and smooth muscle. In innate immune cells such as monocytes and macrophages, they are especially prevalent.
The function of ethanolamine plasmalogens remains obscure, hypotheses concerning their role in arachidonic acid reservoir and protection of other lipids against oxidation (through their vinyl group) were early suggested (Leray C et al., Lipids 2002, 37, 285). It was also suggested that plasmalogens may contribute to maintaining membrane fluidity and ion exchanges in oysters living in acid sea water (Caillon C et al., Biorxix 2023). They are frequently found in specific membrane microdomains called lipid rafts. Their relative content in plasmalogens may affect properties, such as fluidity, tendency to fusion, and density, These plasmalogens at a remarkably low effective dose were shown to significantly ameliorated cognitive deficits in cognitively impaired mice (Yu J et al., J Funct Foods 2025, 128, 106790). This result further emphasized its potential relevance in neurodegenerative disease research.
The antioxidative properties of plasmalogens are based on two electron-free oxidants reacting with the vinyl ether bond, leading to the production of stable products. Thus, the reaction of plasmalogen with HOCl results in the formation of a lysophospholipid and a a-chloro-fatty aldehyde. That α-chlorofatty aldehyde can be further oxidized to a α–chlorofatty acid or reduced to a α-chlorofatty alcohol (Wang WY et al., Anal Biochem 2013, 443, 148). Myeloperoxidase, found in neutrophils, monocytes, and macrophages, converts during activation of these cells hydrogen peroxide into hypochlorous acid (HOCl). HOCl contributes to the antimicrobial and cytotoxic properties of these leukocytes, but may be also involved in the pathogenesis of several diseases.
Plasma lipidomics analyses have reveals that lyso-plasmalogens concentrations displayed the strongest difference between sexes (15.8% higher in men relative to women) (Beyene HB et al. Plos Biol 2020, 18:e3000870).
In contrast, a study of the oxidative stability of soybean oil and egg phospholipid liposomes leads to the conclusion that plasmalogens are not an antioxidant but rather a pro-oxidant (Wang G et al., J Agric Food Chem 2010, 58, 2554).
A function in the mediation of cellular cholesterol efflux has been also discovered (Mandel H et al., Biochem Biophys Res Comm 1998, 250, 369). It was also shown that the elevation of the cellular plasmalogen level reduces cholesterol biosynthesis by enhancing the degradation of squalene monooxygenase (Honsho M et al., J Biol Chem 2015, 290, 28822).
The plasmalogen synthesis via the peroxisomal pathway was shown to decrease with age and more severely in patients suffering Alzheimer disease. A precise link between plasmalogen levels and that pathology remains to be defined while serum plasmalogen level was proposed as a way of predicting cognitive decline in Alzheimer disease patients (Goodenowe DB et al., J Lipid Res 1977, 48, 2485; Wood PL et al., J Psychiatry Neurosci 2010, 35, 59; Su XQ et al. Lipids Health Dis 2019, 18:100)
Ethanolamine-plasmalogens were shown to be reliable biomarker to evaluate malignancy and metastatic capacity of human cancers (Smith RE et al., Lipids 2008, 43, 79). Thus, major changes were observed in the ratio monoenoic/saturated fatty acids which can distinguish with high sensitivity normal tissues from either benign or neoplastic tissues from breast, lung, or prostate lesions.
The depletion of plasmalogens, or their reduced amount, was shown to be associated with chronic lung disease (asthma, chronic obstructive pulmonary disease (COPD), neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease), cardiometabolic disease, and nonalcoholic steatohepatitis.
Plasmalogens appear crucial for innate immune cells to exhibit enhanced responses to bacterial lipopolysaccharide and to effect phagocytosis (Rubio JM et al., Front Immunol 2018, 9, 1723). Furthermore, plasmalogens may be instrumental for the execution of ferroptosis, a nonapoptotic form of cell death associated with oxidative stress (Review in: Balsinde J et al., Biomolecules 2024, 14, 1461). In 2017, phoshatidylethanolamine has been shown to be specifically relevant to ferroptotic cell death (Kagan VE et al., Nat Chem Biol 2017, 13, 81).
A decrease in the biosynthesis of plasmalogens is observed in several peroxisome biogenesis disorders such as the Zellweger syndrome, the neonatal adrenoleukodystrophy and some form of chondrodysplasia (Steinberg SJ et al., Biochim Biophys Acta 2006, 1763, 1733). A possible link between membrane plasmalogen composition and cellular cholesterol regulation has been proposed (Mankidy R et al., Lipids Health Disease 9, 62).
Ether phospholipids have emerged as novel candidates in protecting against alcoholic liver disease based on their unique structural and functional properties. In a murine model of that pathology, plasmenyl phosphatidylethanolamine (and plasmanyl phosphatidylcholine), derived from sea cucumber, attenuated ethanol-induced liver injury, evidenced by reduced lipid accumulation and suppressed ferroptotic hallmarks (Liu Y et al., J Agric Food Chem June 13, 2025). Theser findings unveil marine-food-derived ether phospholipids as novel dietary modulators of ferroptotic damage, offering a dietary strategy for alcoholic liver disease.
For an extensive review of functions and biosynthesis of plasmalogens in health and disease, refer to the paper by Brites P et al. (Brites P et al., Biochim Biophys Acta 2004, 1636, 219).
The appearance, disappearance and reappearance of plasmalogens in evolution, from bacteria to mammals, has been discussed in a review paper (Goldfine H, Prog Lipid Res 2010, 49, 493).
The role of ethanolamine plasmalogens in neurological diseases and in metabolic and inflammatory disorders has been reviewed (Wallner S et al., Chem Phys Lipids 2011, 164, 573).
The alkyl-, acyl-derivative (1-alkyl-, 2-acyl-sn-glycero-3-phosphorylethanolamine) is present in small amount in all phospholipid extracts.
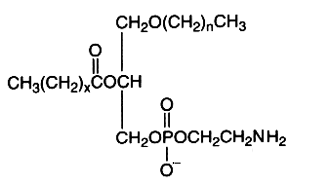
It was first isolated from bovine erythrocytes but is present also in reasonably amount in human lens (Deeley JM et al., Anal Chem 2009, 81, 1920), bone marrow, platelets, certain protozoa and molluscs. Ethanolamine plasmalogens are about 5 times and 4 times more abundant than the diacyl forms in oyster (Crassostrea gigas) and in mussel (Mytilus edulis), respectively (Kraffe E et al., Lipids 2004, 39, 59). These plasmalogens were found to be enriched with non-methylene-interrupted fatty acids (mainly 7,17-22:2).
N-methyl and N,N-dimethyl phosphatidylethanolamine
These groups have either N-methylethanolamine or N,N-dimethylethanolamine substituted for ethanolamine in their structure. Bremer (Biochim Biophys Acta 1959, 35, 287) proposed a possible role of these compounds in interconversions from phosphatidylethanolamine to phosphatidylcholine in rat liver. This was confirmed later and extensively studied in nervous tissues.
N-Methyl phosphatidylethanolamine has been detected in a Gram-positive bacteria Clostridium acetobutylicum (Lepage C et al., J Gen Microbiol 1987, 133, 103).
These phospholipids may be separated from phosphatidylcholine and phosphatidylethanolamine by mass spectrometry (Bilgin M et al., Biochim Biophys Acta 2011, 1811, 1081).
Phosphatidylethanolamine-hydroxy-alkenals
Phosphatidylethanolamine can be covalently modified in vitro and in cellular systems by hydroxy-alkenals, such as 4-hydroxy-2-nonenal (4-HNE; from n-6 fatty acids), 4-hydroxy-2-hexenal (4-HHE; from n-3 fatty acids), and 4-hydroxy-dodecadienal (4-HDDE; from the 12-lipoxygenase product of arachidonic acid), to form PE-alkenal Michael adducts (Guichardant M et al., Free Rad Biol Med 1998, 25, 1049; Bacot S et al., J Lipid Res 2007, 48, 816). Several other bioactive aldehyde-modified phosphatidylethanolamines have been described (Guo L et al., Free Rad Biol Med 2012, 53, 1226). Below, the adduct formed between PE and 4-HHE is shown.
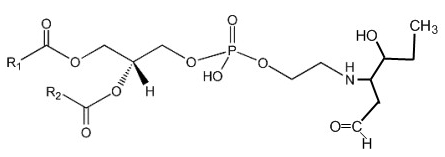
Several experimental results suggest that these adducts could be used as specific markers of membrane disorders occurring in pathophysiological states with associated oxidative stress and might affect cell function.
Phosphatidylethanolamine-bisretinoid (A2-PE)
A2-PE is formed as a byproduct of the visual cycle in the photoreceptor outer segment, its synthesis occurring when molecules of all-trans– retinal, rather than undergoing reduction to retinol, react with phosphatidylethanolamine (PE) (Liu J et al., J Biol Chem 2000, 275, 29354). That compound was tentatively identified by Adams RG in 1967 as a Schiff base of retinal and phosphatidylethanolamine in bovine rod outer segments (Adams RG et al., J Lipid Res 1967, 8, 245).
It has been proved that exposure to bright light favors A2-PE formation in the retina. Furthermore, a close relationship between lipofuscin accumulation and retinal degeneration and juvenile onset recessive Stargardt disease is now evident. The fluorophore A2E is generated by phosphate hydrolysis of its precursor, A2-PE.
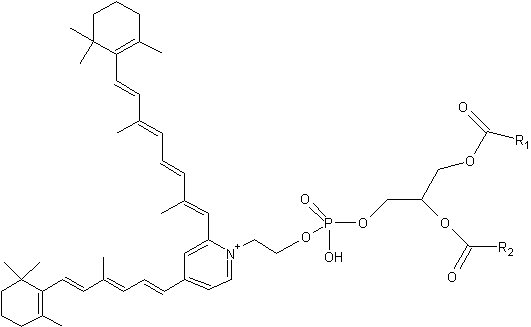
Phosphatidylethanolamine-bisretinoid
R1 and R2 are the fatty acid chains
Isoketal phosphatidylethanolamine adducts
Isoketals (or isolevuglandins) form adduct to phosphatidylethanolamine in vitro (Bernoud-Hubac N et al., Free Radic Biol Med 2004, 37, 1604), whether PE adducts are formed in cells is unknown.
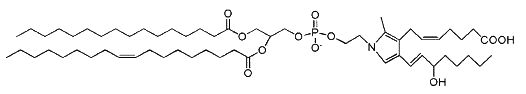
Isoketal phosphotidylethanolamine adduct
It has been demonstrated that these PE adducts dose dependently induced cytotoxicity (LC 50 2.2 mM) in human umbilical vein endothelial cells (Sullivan CB et al., J Lipid Res 2010, 51, 999). Furthermore, isoketal dose-dependently increased PE adduct concentrations to a greater extent than protein adduct. These results indicate that cellular PE is a significant target of isoketals, and that formation of PE adducts may mediate some of the biological effects of isoketal relevant to diseases. Whether these adducts contribute to pathogenesis of diseases associated with inflammation and oxidative stress (atherosclerosis, end stage kidney disease, myocardial infarction, Alzheimer’s Disease, glaucoma) or are simply byproducts of the disease remains an active area of investigation. It has been proposed that isolevuglandin-PE adducts provide a dosimeter of oxidative injury, being potential biomarkers for assessing risk for oxidative stress-stimulated diseases (Li W et al., Free Rad Biol Med 2009, 47, 1539).
It was demonstrated that these PE derivatives could mediate some of the proinflammatory effects of lipid peroxidation, particularly in capillary vessels (Guo L et al., J Biol Chem 2011, 286, 18170).
N-acetyl phosphatidylethanolamine
That unusual phosphatidylethanolamine derivative was described in a filamentous fungus, Absidia corymbifera, and formed about 6% of total membrane lipids (Batrakov SG et al., Biochim Biophys Acta 2001, 1531, 169).
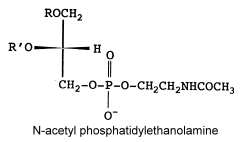
The main fatty acids (R and R’) are 16:0, 18:0 and 18:2
N-ethoxycarbonyl phosphatidylethanolamine
Another unusual PE derivative was, as the previous one, isolated from Absidia corymbifera, N-ethoxycarbonyl phosphatidylethanolamine and formed about 9% of total membrane lipids (Batrakov SG et al., Biochim Biophys Acta 2001, 1531, 169).
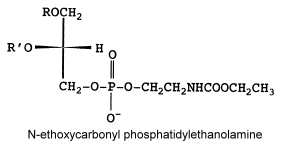
The fatty acid pattern is similar to N-acetyl PE but also with a prominent proportion of 18:3.
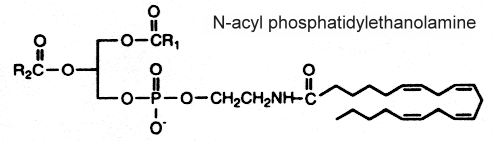
N-acyl phosphatidylethanolamine
This complex lipid containing three fatty acid chains was isolated in 1965 by Bomstein RA (Biochem Biophys Res Comm 1965, 21, 49) from wheat flour. Later, it was shown to be present in quite all grains (pea seeds, oats and soya beans) but its presence was also reported in microorganisms, fish, and in mammalian tissues (Schmid HHO et al., Prog Lipid Res 1990, 29, 1). In oat lipids the major N-acylated fatty acids are 16:0, 18:2 and 18:1 (Holmbäck J et al., Lipids 2001, 36, 153-165). In animal cells (heart, brain, liver and skeletal muscle), the N-acyl chain is frequently palmitic or stearic acid. Very long-chain anteiso alkanoic acids (C20 to C35) have been described in N-acylPE of a mat-forming cyanobacterium (Calothrix sp.) collected from a lake in James Ross Island (Antarctica) (Rezanka T et al., Phytochemistry 2009, 70, 655).
The mechanism proposed for the synthesis of this complex phospholipid includes the action of an N-acyl transferase catalyzing an exchange reaction between the sn-1 position of phospholipids (probably phosphatidylcholine) and the primary amine of phosphatidylethanolamine.
Several reports indicate the importance of N-acyl phosphatidylethanolamine as precursors for N-acylethanolamines, which in turn play a physiological role during germination of seeds (Chapman KD et al., Plant Physiol 1999, 120, 1157) and in defense systems in plants (Tripathy S et al., Plant Physiol 1999, 121, 1299). The biosynthesis and functions of N-acyl phosphatidylethanolamine in plants has been reviewed (Coulon D et al., Biochimie 2012, 94, 75).
The biological functions of the N-acylation of phosphatidylethanolamine in mammals have been reviewed (Wellner N et al., Biochim Biophys Acta 2013, 1831, 652).
In addition to be a source of acylethanolamines, N-acyl phosphatidylethanolamine was shown to be secreted in the rat into circulation from the small intestine in response to ingested fat and that systemic administration of the most abundant circulating N-acyl phosphatidylethanolamine (C18 acylated), at physiologic doses, decreases food intake (Gillum MP et al., Cell 2008, 135, 813). Metabolic studies have shown that its effects may be mediated through direct interactions with the central nervous system. These acylated phospholipids may be the base of future therapeutic agents for the treatment of obesity.
N-Acylated ethanolamines were first isolated from preparations of egg yolk, peanut oils, and soybeans due to their anti-inflammatory and anti-allergienic properties (Kuehl FA et al., J Am Chem Soc 1957, 79, 5577). Interestingly, a unique N-acyl phosphatidylethanolamine was proposed in the brain as a source of N-arachidonoyl ethanolamine (named also anandamide, from the Sanskrit “ananda” that means bliss), this metabolite appears to be formed (with a molecule of phosphatidic acid) by phospholipase D action. Further investigations provide evidence for a multistep pathway by the sequential actions of hydrolases and glycerophosphodiesterase (Simon GM et al., J Biol Chem 2008, 283, 9341).
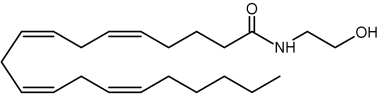
Anandamide
This component (known as endocannabinoid), identified in the porcine brain, behaves like an endogenous cannabinoid (Devane et al., Science 1992, 258, 1946) and is now considered as the main ligand of the cannabinoid receptor discovered in 1988 in the rat brain (Devane WA et al., Mol Pharmacol 1988, 34, 605) and thereafter in various types of cells. It was determined that it has essential properties to be classified as a cannabinoid/anandamide receptor agonist (Felder CC et al., PNAS 1993, 90, 7656). Intense research are made at present in this field (Review in: Hillard CJ et al., J Lipid Res 1997, 38, 2383; Bezuglov VV et al., Biochemistry, Moscow 1998, 63, 22). Numerous roles have been proposed for the involvement of anandamide in fertilization, blastocyst development, implantation, pregnancy maintenance, and parturition. Many observations support the hypothesis that low systemic levels of anandamide are required for normal pregnancy progression (Maccarrone M et al., Mol Hum Reprod 2002, 8, 188). Anandamide was reproducibly quantified in human placenta, cord blood and fetal membranes using solid-phase extraction combined with liquid chromatography tandem mass spectrometry (Marczylo TH et al., Anal Biochem 2010, 400, 155).
N-Acylethanolamines have been reported to exhibit neuroprotective properties in models of ischemic strokes (Sinor AD et al., Neurosci Lett 2000, 278, 157).
Recent insights on intracellular stores and anandamide binding proteins call for a reconsideration of the dogma that endocannabinoids are exclusively synthesized and released “on demand”, and suggest that their metabolic control is complemented by intracellular transport and storage in specific reservoirs (adiposomes) (Maccarrone M et al., Tr Biochem Sci 2010, 35, 601).
Vaccenic acid has been shown to attenuate complications observed in the metabolic syndrome, including dyslipidemia, fatty liver disease, and low-grade inflammation. An explanation of that property could be that vaccenic acid lowers intestinal inflammation by increasing the production of anandamide and related N-acylethanolamines (Jacome-Sosa M et al., J Lipid Res 2016, 57, 638).
Two new ethanolamides were isolated from porcine brain and shown to be ligands of the cannabinoid receptors CB1 : dihomo-γ-linolenoylethanolamine and docosatetraenoylethanolamine ( Hanus L et al., J Med Chem 1993, 36, 3032; Pertwee R et al., Eur J Pharmacol 1994, 259, 11). It is also possible that a palmitoylethanolamine exists in brain tissue.
Because neural tissue is rich in n-3 polyunsaturated fatty acids, especially docosahexaenoic acid (DHA, 22:6 n-3), it was suggested that they could be present in acylethanolamides in the brain (Hanus L et al., J Med Chem 1993, 36, 3032). It was later shown that this DHA-derived structural analog of an anandamide has a number of bioactive functions such as neuritogenesis and synaptogenesis (Kim HY et al., Biochem J 2011, 435, 327). These properties led to the proposition of the term “synaptamide” to indicate its synaptogenic properties and chemical nature (Kim HY et al., Prostagl Other Lipid Mediators 2011, 96, 114). The brain synaptamide content is dependent on the dietary DHA intake, suggesting an endogenous mechanism whereby diets containing adequate amounts of omega-3 fatty acids improve synaptogenesis in addition to well-recognized anti-inflammatory effects (Kim HY et al., Prost Leukotr Essential Fatty acids 2013, 88, 121).
A novel N-acylethanolamine, tetracosahexaenoylethanolamide (THEA) was shown to be present in the mice brain and that its level was higher upon ischemia/hypercapnia and by KO of the fatty acid amide hydrolase enzyme. While THEA did not activate PPAR, it augmented the excitability of medium spiny neurons in the nucleus accumbens. Overall, the results suggest that THEA regulates neuronal excitation (Lin L et al., J Lipid Res 2020, 61, 1480). One of Tetracosahexaenoic acid (THA, 24:6n-3), was first identified as an unstable precursor to DHA in 1991, but was later determined to also be a product of DHA.
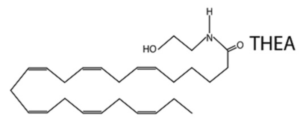
It has been clearly determined that endocannabinoids are involved in the regulation of food intake and body energy reserves (control of energy homeostasis) and they participate to pathological mechanisms leading to various metabolic dysfunctions (Review: Kunos G et al., J Biol Chem 2008, 283, 33021).
A review on the biological activities, pharmacology, and signal transduction mechanisms for the cannabinoid receptors has been released (Howlett AC, Prost Lipid Med 2002, 68-69, 619). Palmitoylethanolamide and stearoylethanolamide are known for their anti-inflammatory properties (Costa B et al., Pain, 2008, 139, 541; Dalle Carbonare M et al., J Neuroendocrinol 2008, 20, 26).
Palmitoylethanolamide has been shown to work synergistically with the endocannabinoid, anandamide (Ho WS et al., Br J Pharmacol 2008, 155, 837). Palmitoylethanolamide was also proposed to have an effect on sleep due to its ability to act through transient receptor potential cation channel subfamily V member 1 (TRPV1). New findings support that this compound could be a potential sleeping aid capable of reducing sleep onset time and improving cognition on waking (Rao A et al., Sleep Science and Practice 2021, 5, 12). Later findings encourage also the use of palmitoylethanolamide as a potentially effective therapy in patients with long COVID (Raciti L et al., Med Sci (Basel) 2022, 10(3):37).
Oleoylethanolamide has been described to have anorexogenic properties (Schwartz GH et al., Cell Metab 2008, 8, 281). Furthermore, all N-acylethanolamines activate PPAR-a (Sun Y et al., Biochem Soc Trans 2006, 34, 1095). Short-term feeding experiments have shown that the levels of N-acylethanolamines could be affected (Artmann A et al., Biochim Biophys Acta 2008, 1781, 200). It has been proposed a model by which the released ethanolamine contributes to the overall balance between dopamine and acetylcholine after oleoylethanolamide administration (Mennella I et al., J Nutri 2019, 149, 362).
Details on the pharmacology of these compounds are found on Pharmacology Central. A recent review on bioactive amides of fatty acids is found on the site of Biochimica (Moskov) and in a review article (Di Marzo V et al., Lipids 1999, suppl 34, S319). An extensive review of the endocannabinoid system, with its targets, main compounds and therapeutic applications, may be consulted (Lambert DM et al., J Med Chem 2005, 48, 5059). A review discusses evidence that the endocannabinoid system constitutes a new physiological pathway occurring in the central nervous system and peripheral tissues that has a key role in the control of food intake and energy expenditure, insulin sensitivity, as well as glucose and lipid metabolism (André A et al., Int J Biochem Cell Biol 2010, 42, 1788).
A review on the determination and quantitation of fatty acid amides was released by Walker JM et al. (Prost Lipid Med 2005, 77, 35). A quantitative method for the profiling of the various endocannabinoids present in rat brain by mass spectrometry has been described (Williams J et al., Anal Chem 2007, 79, 5582).
The physiological properties and the therapeutic opportunities of anandamide and other N-acylethanolamines have been reviewed (Mock ED et al., Prog Lipid Res 2023, 89, 101194).
A comprehensive review on the occurrence, metabolism and functions of this bioactive class of lipid mediators in plants was written by Chapman KD (Prog Lipid Res 2004, 43, 302) and an overview of the biochemistry and pharmacology of anandamide has been released (Hansen HS et al., Eur J Lipid Sci Technol 2006, 108, 877). In plants, N-acylethanolamines are thought to be involved in several physiological processes such as the regulation of seed germination, elongation of roots, seedling growth, flower senescence, plant defence against pathogen attack and inhibition of stomatal closure (Kim SC et al., Plant Sci 2010, 178, 411).
A comprehensive graphical description of the metabolism and the signaling process of anandamide may be found on a specific web site.
O-Arachidonoyl ethanolamine with an ester instead of an amide linkage was isolated from brain (see virodhamine).
Evidence was accumulated in 1997 that cyclooxygenase (COX-2) recognizes anandamide and catalyzes its conversion to a new compound, prostaglandin E ethanolamide, belonging to a new class of prostaglandins, prostaglandin ethanolamines or prostamides (Yu M et al., J Biol Chem 1997, 272, 21181).
Similarly, it has been shown that in plants N-linoleyl ethanolamide can be converted by a 13-lipoxygenase into the corresponding lipoxin Shrestha R et al. Plant Physiol 2002, 130, 391).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire