
In his classic studies on fatty acids from pork fat, Chevreul (1823) recognized the nature of oleic acid but it was not prepared in pure condition for a long time. Its structure was not definitively elucidated by a series of complex chemical reactions until later (Baruch J, Ber 1894, 27, 172). Much simpler proof of the now accepted structure of oleic acid was given by means of oxidation techniques (Edmed FG, J Chem Soc 1898, 73, 627). The ozonisation method for the determination of the position of the unsaturated linkage was used for the first time in lipidology in 1903 (Molinari E, Annuario della Soc Chimica di Milano 1903, 9, 507). Oleic acid synthesis was realized in 1934 (Noller CR et al., J Am Chem Soc 1934, 56, 1563).
Mono-unsaturated normal fatty acids are widespread in the living world where they occur mostly as the cis-isomer. Over a hundred naturally occurring monoene fatty acids have been identified. They have the general structure:
CH3(CH2)xCH=CH(CH2)yCOOH
The most frequently they have an even number of carbon atoms and the unique double bond may be in a number of different positions.
The double bond can exist in two stereoisomeric forms :
![]()
The commonest cis-monoenes are of the n-9 series, as oleic acid from olive oil (cis-9-octadecenoic acid) and from quite all seed oils.
Some important monoenoic acids are found below:
|
Systematic name |
Trivial name |
Shorthand designation |
Molecular wt. |
Melting point (°C) |
| cis-4-decenoic | obtusilic | 10:1 (n-6) | 170.3 | |
| cis-9-decenoic | caproleic | 10:1 (n-1) | 170.3 | |
| cis-5-dodecenoic | 5-lauroleic (denticetic) | 12:1 (n-7) | 198.4 | |
| cis-4-dodecenoic | linderic | 12:1 (n-8) | 198.4 | |
| cis-9-tetradecenoic |
myristoleic |
14:1 (n-5) |
226.4 |
– |
| cis-5-tetradecenoic | physeteric | 14:1 (n-9) | 226.4 | |
| cis-4-tetradecenoic | tsuzuic | 14:1 (n-10) | 226.4 | |
| cis-9-hexadecenoic |
palmitoleic |
16:1 (n-7) |
254.4 |
0.5 |
| cis-6-hexadecenoic | sapienic | 16:1 (n-10) | 254.4 | |
| cis-6-octadecenoic |
petroselinic |
18:1 (n-12) |
282.4 |
30 |
| cis-9-octadecenoic |
oleic |
18:1 (n-9) |
282.4 |
16.2 |
| tr-9-octadecenoic | elaidic | tr18:1 (n-9) | 282.4 | 43.7 |
| cis-11-octadecenoic |
vaccenic (asclepic) |
18:1 (n-7) |
282.4 |
39 |
| cis-9-eicosenoic |
gadoleic |
20:1 (n-11) |
310.5 |
25 |
| cis-11-eicosenoic |
gondoic |
20:1 (n-9) |
310.5 |
– |
| cis-11-docosenoic | cetoleic | 22:1 (n-11) | 338.6 | |
| cis-13-docosenoic |
erucic |
22:1 (n-9) |
338.6 |
33.4 |
| cis-15-tetracosenoic |
nervonic |
24:1 (n-9) |
366.6 |
39 |
While this common fatty acid is mainly found acylated in glycerides, it may be found sometimes as ethyl esters in organs of animal treated with ethanol (Hungund BL et al., J Chem Pharmacol 1988, 37, 3001) and may serve as markers of ethanol intake. (Laposata M, Prog Lipid Res 1998, 37, 307).
The first synthesis of oleic acid appeared in the literature in 1934 (Noller CR et al., J Am Chem Soc 1934, 56, 1563).
Monoethanolamine oleate (ethanolammonium oleate) is a salt formed by the reaction between monoethanolamine and oleic acid. It is used as a sclerosing agent in chronic venous disorders (Rabe E et al., Phlebology. 2014, 29, 338).
Ethyl oleate was identified as a primer pheromone in honey bees in causing a delayed onset of foraging in younger individuals (Leoncini I et al., PNAS 2004, 101, 17559).
In 2010, the Mediterranean diet was recognized by UNESCO as an Intangible Cultural Heritage of Humanity. Olive oil is the most characteristic food of this diet due to its high nutraceutical value and its positive effects have often been attributed to oleic acid (70-80%) but also to its minor component such as oleoylethanolamide with anti-inflammatory and antioxidant effects. That compound derived from oleic acid is an endogenous ligand of the peroxisome proliferator-activated receptor alpha (PPARα) nuclear receptor. Thus, it may regulate dietary fat intake and energy homeostasis (Review in: Santa-María C et al., Nutrients 2023, 15, 224).
N-1 monoenes : 8-Nonenoic Acid was shown to be emitted by Kocuria flava, a bacterial endophyte of the marine macroalga Bryopsis plumosa. This fatty acid has the property to inhibit the aquaculture pathogen Saprolegnia parasitica (Deutsch Y et al., Mar Drugs 2023, 21, 476). This compound could enable managing oomycete agricultural pathogens in general, and S. parasitica in particular, a major causal agent in aquaculture diseases.
N-3 monoenes : An unusual isoform, the lauroleic acid (9-dodecenoic acid, 12:1 n-3) has been described as a natural metabolite of lauric acid (12:0) in rat hepatocytes (Legrand P et al., Lipids 2002, 37, 569)
N-5 monoenes : Seeds of Androsace septentrionalis (Primulaceae) were shown to contain an unusual fatty acid : 16:1 n-5 (Tsevegsuren N et al., Lipids 2003, 38, 1173). That fatty acid, together with 18:1 n-5, were describeed in deep-sea Foraminifera (Kharlamenko VI et al., Lipids 2017, 52, 345).
N-7 monoenes : The 5-dodecenoic acid (denticetic acid, 12:1 n-7) is present in sperm whale oil. This compound was formerly known to be present in milk lipids.
The rare 21:4 n-7 has been described in some animal species (crab, sponge, protist), but it was found in high amount (about 9 % of the total fatty acids) in a opisthobranch mollusc, Scaphander lignarius (Vasskog T et al., Mar Drugs 2012, 10, 2676). That fatty acid was shown to be cytotoxic against human cancer cell lines normal lung fibroblasts, more than EPA and arachidonic acid.
N-8 monoenes : An isomer (12:1 n-8, linderic acid) has been found as the major fatty acid (47%) in the seed oil of a Lauraceae, Lindera umbellata (Hopkins CY et al., Lipids 1961, 1, 118). This fatty acid is not known to occur in any other plant family.
N-10 monoenes : Sapienic acid (16:1 n-10), a 16-carbon fatty acid with a single cis double bond at the sixth carbon from the carboxyl end, is the most abundant fatty acid in human wax sebum, and among hair-bearing animals is restricted to humans (Nicolaides N, Science 1974, 186, 19). Sapienic acid has been also identified in in human hair and nail samples beside 18:1n-10 (Destaillats F et al., J Chromatogr A 2011, 1218, 9384). Thus this fatty acid is truly unique to sebum, hair and nail and is not found anywhere else in the human body.
Notably, this fatty acid has been implicated in the pathogenesis of acne (Downing DT et al., J Am Acad Dermatol 1986, 14, 221) but was shown to be effective against gram-positive bacteria in human skin sebum (Wille JJ et al.., Skin Pharmacol Physiol 2003, 16, 176). Further works characterized its biosynthesis by a Δ-6 desaturase acting on palmitic acid (Ge L et al., J Invest Dermatol 2003, 120, 707). While sapienic acid is unique to humans in the animal kingdom, it is not unique in life. It is the major fatty acid of certain plant seeds, such as Thunbergia alata of the Acanthaceae family (Spencer GF et al., Lipids 1971, 10, 712). Sapienic acid has been also detected in deep-sea Foraminifera (Kharlamenko VI et al., Lipids 2017, 52, 345).
N-12 fatty acids : 16:1 n-12 has been detected in lipid extracts from deep-sea Foraminifera (Kharlamenko VI et al., Lipids 2017, 52, 345).
N-13 fatty acids : The acid 20:1n-13 is prominent in lipids of the Ophiuroidea (5–24%) and Asteroidea (15–20%), and it is not found in the Crinoidea. The distribution of this lipid depends on the taxonomic position of the species. A high level of 20:1n-13 has been recorded from the coastal brittle star Amphiura elandiformis (8.7%) (Mansour MP et al., Biochem Syst Ecol 2005, 33, 659).
It has been suggested that by GC chromatography the 20:1n-9, reported in other papers on brittle stars and other echinoderms, may be mainly the 20:1n-13 isomer.
N-14 fatty acids : 21:1 n-14 has been detected in lipid extracts from deep-sea Foraminifera (Kharlamenko VI et al., Lipids 2017, 52, 345).
N-15 fatty acids : An unusual 20 carbon fatty acid (20:1 n-15) is found in high concentration (about 60%) in seeds of Limnanthes alba (meadowfoam), an herbaceous winter annual plant native to the pacific Northwest area of the United States.
No fatty acid exists naturally with the double bond close to the terminal methyl group, only one is chemically synthesized from ricinoleic acid, the undecylenic acid (11:1 n-10).
![]()
One geometrical and several positional isomers of oleic acid exist with a trans double bond. Among the naturally occurring trans isomers, the double bond is in the (n-13), (n-12), (n-9) or (n-7) position.
Vegetable oils and fats are almost trans free, if not warmed at high temperature in the presence of active components. In contrast, trans fatty acids occur in most animal fats especially in butter and ruminant fats.
Among the most common, Elaidic acid (t9-octadecenoic acid) and t-Vaccenic acid (t11-octadecenoic acid) are found in the rumen and in lipids of ruminant animals. trans-Vaccenic acid which is the major trans-monounsaturated fatty acid present in several food products (milk, yoghurt, cheese, butter and meats) results from the bio-hydrogenation of rumenic acid.
The elaidinization reaction was first obtained by a French pharmacist and chemist, Jean-Joseph-Étienne Poutet (1779-1858) (Ann Chim Phys 1819, 12, 58), who observed that trioleine could be converted to the consistency of pork lard when treated with the oxides of nitrogen derived from mercurous nitrate (mainly nitrous acid).

Later, Boudet F (Ann Chim Phys 1832, 50, 391; J Pharm 1832, 18, 469) studied accurately the reaction and isolated after saponification of “elaidine” (obtained from triolein) a fatty acid melting at 36°C which he named “acide élaidique” (elaïdic acid), from the Greek name of olive (elais, elaidos).

Until 1952, elaidic acid was known only as a laboratory isomerization product of oleic acid. This trans fatty acid was demonstrated by infrared analysis to be present in substantial quantities in beef fat (Swern D et al., JAOCS 1952, 29, 44). Later, it was shown that trans fatty acids arise in the first stomach of ruminants as products of catalytic hydrogenation of dietary unsaturated fatty acids (conjugated by an isomerase) during bacterial fermentation. As a result, butter, cheese, milk, beef and mutton fats contain approximately 2-8% transfatty acids by weight.
Trans-fatty acids are also formed in varying amounts during the industrial hydrogenation of plant or fish oils. This hydrogenation improves the thermal stability and prevents any oxidative process in very unsaturated oils. The natural kick found in cis-fatty acids disappears and the molecule becomes linear and thus has physical properties similar to saturated fatty acids as it was observed by Poutet in 1819.
Trans-fatty acids are also formed under the action of thiyl radicals.
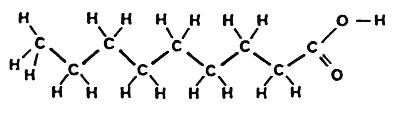
Saturated fatty acids
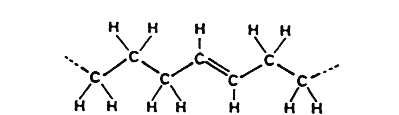
trans-Fatty acid
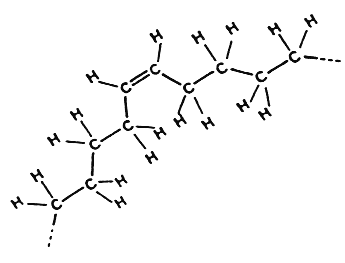
cis-Fatty acid
An unusual trans fatty acid, t3-hexadecenoic acid (trans-16:1 n-13), occurs in eukaryotic photosynthetic membranes (mainly in phosphatidylglycerol) from higher plants and green algae. As this fatty acid is absent from etiolated tissue, it has been inferred that it has a specific role associated with the light reactions of photosynthesis (Gounaris K et al., Biochem J 1986, 237, 313).
trans Fatty acids are formed by some bacteria (predominantly gram negative and under anaerobic conditions) via double-bond migration and isomerization.
The predominant 18:1 trans isomers in partially hydrogenated vegetal oils have their double bond in position t9, t10, t11 and t12, but their distribution (10-22% for t8, t9, t10, t11, t12 or t13 isomer) is distinct from that of milk fat, which contains (2-6% of the total fatty acids) vaccenic acid (t11-18:1) as the predominant isomer (about 60% of t11 and 4-8% for each of the others). The trans-18:1 acid contents of beef meat fat and tallow are about 2 % and 5 %, respectively. Its presence in ruminant fats is explained by a biohydrogenation of linoleic acid occurring in the rumen.
The trans isomers account for about 4.5 % of total fatty acids in ewe milk fat and 3 % in goat milk fat. While the contribution of these two milk sources may be estimated as negligible in most EEC countries, in Greece ewe and goat milk fat contribute for about 45 % of the daily consumption of vaccenic acid.
The daily per capita intake of trans-18:1 acids from ruminant fats was estimated to be about 1.5 g for people from most countries of the EEC, Spain and Portugal being exceptions (about 0.8 g/person/day) (review in Wolff RL, AOCS 1995, 72, 259). An estimation of the trans fatty acid content of foods and intake levels in France has been reported in 2007 (Laloux L et al., Eur J Lipid Sci Technol 2007, 109, 918). The trans fatty acid intakes and their food sources have been determined in the U.S. population (Kris-Etherton PM et al., Lipids 2012, 47, 931).
It is not yet known what effect continuous consumption and exposure to trans fatty acids isomers will have on human health, specifically those present in ruminant fats, deodorized vegetable oils, frying oils, and those present in synthesized products (Aldai N et al., Eur J Lipid Sci technol 2013, 115, 1378). In rats, it was demonstrated that there is a direct retroconversion of dietary trans-vaccenic acid into trans-palmitoleic acid. As it was shown that circulating trans-palmitoleic acid level is associated in man with a lower risk of metabolic syndrome (Kalergis M et al., Front Endocrinol 2013, 4:90), there is a need of deeper knowledge about the origin of that fatty acid and its impact on health. An overview was provided about trans fatty acids origins, their use and consumption among French population with a discussion on their potential human health implications as well as the preventive and regulatory measures undertaken in France (Menas F et al., Eur J Nutr 2013, 52,1289).
The relationships between eighteen‑carbon trans fatty acids and inflammation in the context of atherosclerosis has been reviewed (Valenzuela CA et al., Prog Lipid Res 2019, 76, 101009). Briefly, the evidence associating industrial trans fatty acids with cardiovascular diseases risk factors is fairly consistent, in humans there is a clear a relation between these trans fatty acids and higher levels of inflammatory markers. In contrast, studies in humans, animals and in vitro suggest that ruminant (natural) trans fatty acids have null or mildly beneficial effects in cardiovascular health, metabolic parameters and inflammatory markers.
A review focuses on the mechanisms that underlie the deleterious effects of trans fatty acids by comparing the effects of trans fatty acids to those of cis-unsaturated fatty acids and saturated fatty acids (Oteng AB et al., Adv Nutr 2019 Nov 29). This review also carefully explores the argument that ruminant trans fatty acids have differential effects from industrial trans fatty acids. New data on the molecular mechanisms of action of trans fatty acids may lead to new therapeutic ways for the treatment of diseases characterized by disrupted lipid metabolism. A review has focused on the mechanisms that underlie the deleterious effects of trans fatty acids by juxtaposing effects of trans fatty acids against those of cis-unsaturated fatty acids and saturated fatty acids (Oteng AB et al., Adv Nutr 2020, 11, 697). This review also carefully explores the argument that ruminant trans fatty acids have differential effects from industrial trans fatty acids.
Vaccenic acid has been shown to attenuate complications observed in the metabolic syndrome, including dyslipidemia, fatty liver disease, and low-grade inflammation. An explanation of that property could be that vaccenic acid lowers intestinal inflammation by increasing the production of anandamide and related N-acylethanolamines (Jacome-Sosa M et al., J Lipid Res 2016, 57, 638).
A review of the diversity of adverse health effects of individual trans fatty acid isomers may be consulted (Gebauer SK et al., Lipids 2007, 42, 787). A review of the possible effects of trans fatty acids on heart health and the recommendations for the UK population has been reported (Denny AR, Nutr Bull 2008, 33, 124). While the effects of most of trans fatty acid from ruminants are poorly established, there is increasing evidence that high content of industrial trans fatty acids may cause deleterious effects on human health and life span. A background review paper of their use, consumption, health implications and regulation in France has been published (Menaa F et al., Eur J Nutr 2013, 52, 1289).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire