
These fatty acids (also called polyunsaturated fatty acids, PUFA) have 2 or more cis double bonds which are the most frequently separated from each other by a single methylene group (methylene-interrupted polyenes). Linoleic acid is a typical member of this group. Some rare polyenoic fatty acids may have also a trans double bond. A graphical chart of the oxidation of polyunsaturated fatty acids by free radicals may be found on Wikipedia web site.
-C-C=C-C-C=C-
methylene-interrupted double bonds
Some other polyunsaturated fatty acids undergo a migration of one of their double bonds which are not again methylene-interrupted and are known as conjugated fatty acids.
-C-C=C-C=C-C-
conjugated double bonds
Some unusual fatty acids have not the regular structure with a methylene group between two double bonds but are polymethylene-interrupted polyenes (known also as non-methylene-interrupted fatty acids). They are found in certain classes of bacteria, plants, marine invertebrates and insects.
-C=C-C-C-C-C=C-
polymethylene-interrupted double bonds
Rare fatty acids have allenic double bonds. They are found in some higher plants.
-C=C=C-
allenic double bonds
Very rare fatty acids have cumulenic double bonds. They are present in some higher plants.
-HC=C=C=CH-
cumulenic double bonds
![]()
The most important fatty acids can be grouped into 3 series with a common structural feature: CH3(CH2)xCH=R . x=4 for the (n-6) series and x=1 for the (n-3) series and x=7 for the (n-9) series.
Some rare fatty acids have other structural features.
Below, as an example, we give the structure of a common polyene of the (n-3) series having the double bonds in the 5, 8, 11, 14, and 17 positions (eicosapentaenoic acid or osbond acid).
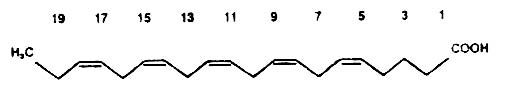 Eicosapentaenoic acid
Eicosapentaenoic acid
The most common polyenoic fatty acids are listed below:
|
Systematic name |
Trivial name |
Shorthand designation |
Molecular wt. |
MP |
| 9,12-octadecadienoic | linoleic acid |
18:2(n-6) |
280.4 |
-5 |
| 6,9,12-octadecatrienoic | γ-linolenic acid |
18:3(n-6) |
278.4 |
|
| 5,11,14–eicosatrienoic | sciadonic acid |
20:3(n-6) |
306.5 |
|
| 8,11,14-eicosatrienoic | dihomo-γ-linolenic acid |
20:3(n-6) |
306.5 |
|
| 5,8,11,14-eicosatetraenoic | arachidonic acid |
20:4(n-6) |
304.5 |
-50 |
| 7,10,13,16-docosatetraenoic |
– |
22:4(n-6) |
332.6 |
|
| 4,7,10,13,16-docosapentaenoic |
osbond acid or n-6 DPA |
22:5(n-6) |
330.6 |
|
| 9,12,15-octadecatrienoic | α-linolenic acid |
18:3(n-3) |
278.4 |
-11 |
| 6,9,12,15-octadecatetraenoic | stearidonic acid |
18:4(n-3) |
276.4 |
-57 |
| 8,11,14,17-eicosatetraenoic |
ETA |
20:4(n-3) |
304.5 |
|
| 5,8,11,14,17-eicosapentaenoic | EPA |
20:5(n-3) |
302.5 |
-54 |
| 7,10,13,16,19-docosapentaenoic | DPA or clupanodonic acid |
22:5(n-3) |
330.6 |
|
| 4,7,10,13,16,19-docosahexaenoic | DHA or nervonic acid |
22:6(n-3) |
328.6 |
-44 |
| 6,9,12,15,18,21-tetracosahexaenoic | nisinic acid | 24:6(n-3) | 356.6 | |
| 5,8,11-eicosatrienoic | Mead acid |
20:3(n-9) |
306.5 |
N-6 FATTY ACIDS
It was isolated in 1844 by Sacc (Ann 1844, 51, 213), and after a long controversy its exact structure was clarified in 1939 (Hilditch TP et al., J Soc Chem Ind 1939, 58, 233) and it was synthesized only in 1950 (Raphael RA et al., Nature, 1950, 165, 235).
It cannot be synthesized by animals which must find it in plant foodstuff. It is said an essential fatty acid for animals. Walnut, peanut, seeds of sunflower, grape, corn, sesame and soya contain large amounts of that fatty acid. Linoleic acid is the precursor of all the (n-6) series formed by desaturation and elongation.
Two trans isomers of linoleic acid have been detected in seed oils. The 9c,12t isomer (M.P. = -5°C) was found in Crepis rubra and the 9t,12t isomer (M.P. = 29°C) was found in Chilopsis linearis.
Linoleic (and arachidonic acid), has been shown to be either pro- or anti-inflammatory, and researchers have advocated both for and against reducing their dietary intake. In a large community-based study, it has been observed a weak but statistically significant inverse associations between several types of inflammatory biomarkers with red blood cell n-6 fatty acids. To date, findings do not support the hypothesis that omega-6 fatty acids are pro-inflammatory (Lai HT et al., Nutrients 2025, 17(13), 2076). More importantly, it must be emphasized that several meta-analyses have assessed the importance of the ratio of n-6 and n-3 fatty acids on the incidence of several pathologies as cardiovascular diseases (Cao M et al., Food Biosci 2024, 59, 104066).
An interesting review of the relationships between biochemistry, nutritional and epigenetic properties of polyunsaturated fatty acids may be consulted (Benatti P et al., J Am College Nutr 2004, 23, 281).
It has been reported that linoleic acid is the most abundant polyunsaturated fatty acid (0.45-2.7g/100g fresh insect, 9-21% of total FA) in insect body (Yang LF et al., J Food Lipids 2006, 13, 277-285).
It was first isolated in 1940 from phospholipids from beef suprarenal glands by Shinowara GY et al. (J Biol Chem 1940, 134, 331) and its structure was elucidated three years later by Arens CL et al. (Biochem J 1943, 37, 1). The first total synthesis of arachidonic acid was made in 1961 (Osbond JM et al., J Chem Soc 1961, p.2779).
Rare in the plant kingdom, it can be found in some fungi, mosses and ferns but is a major component of several microalgae and some marine brown algae. It was shown to be abundant in a green alga, Parietochloris incisa, where it reaches up to 47% of the triglyceride pool (Bigogno C et al. Phytochemistry 2002, 60, 497). Unlike higher plants, mosses contain substantial levels of arachidonic acid, protonema cells containing 20-40% of this compound (Gellerman JL et al., Biochim Biophys Acta 1975, 388, 277). The production of arachidonic acid by microorganisms (fungi, microalgae) has been reviewed by Ratledge C (Structured and modified lipids, Gunstone FD Ed, M Dekker, NY 2001, p. 351). In the 1960s certain fungi were found to have lipids with a high content of arachidonic acid (more than 50% of the total fatty acids). Mortierella alpina (Zygomycetes) was selected for the development of an industrial fermentation process (Suntory in Japan, Martek in USA, and DSM in The Netherlands). An extensive overview of process investigations related to microbial and microalgae productions of arachidonic acid (and g-linolenic acid) may bee consulted (Owen PW et al., Proc Biochem 2005, 40, 3627).
The production of arachidonic acid in transgenic plants which might lead to a sustained source of that fatty acid for use in human and animal food was reviewed by Domergue F et al. (Trends Plant Sci 2005, 10, 113). Its current commercial application is the supplementation of infant formula.
It has been shown that in normal cells the other metabolic pathway beginning by the 18:2n−6 elongation to 20:2n−6 and then the Δ5 desaturation to yield 5Z,11Z,14Z–20:3 (sciadonic acid) rather than 5Z,8Z,11Z,14Z–20:4 (arachidonic acid) has a noticeable inefficiency. It has been demonstrated that in hormone positive breast cancer cells that pathway is more important, thus warranting a larger study to evaluate the importance of sciadonic acid biosynthesis and its functional implication (Park HG et al., Prost Leukotr Essent Fatty Acids 2018, 134, 1–6).
Oxidations of arachidonic acid by reactive oxygen radicals generate several oxidized lipids known as iso-eicosanoids (isoprostanoids and isoleukotrienes). A unique family of free radical-generated derivatives generated by NO2-mediated isomerization of arachidonic acid were described (Jiang H et al., J Biol Chem 1999, 274, 16235). Several isomers (named trans-arachidonic acid) were observed and appeared to have one trans-bond and three cis-bonds. Thus, four such isomers of arachidonic acid can potentially be generated.
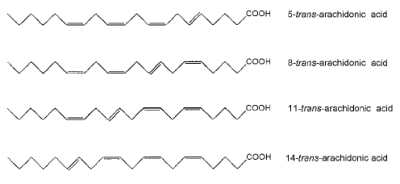
The detection and quantification of trans-arachidonic acids in vivo may be used as a specific index to assess the degree of cellular injury mediated by NO2 since these isomers were shown to be produced in human blood plasma (Zghibeh CM et al., Anal Biochem 2004, 332, 137).
Docosadienoic acid (22:2 n-6 or 13-cis, 16-cis-docosadienoic acid) is a very long chain polyunsaturated fatty acid that has been detected at a high level (up to 57% in seed oil) in Eranthis hyemalis (Ranunculaceae) (Aitzetmüller K, Lipids 1996, 31, 201). That fatty acid has been shown to possess strong anti-inflammatory and antitumor properties (Chen Y et al., Nutr Cancer, 2021, 73, 1697). Improved production of this fatty acid in Brassica carinata crop using biotechnology is providing a real commercial opportunity for high value agriculture products for nutraceutical uses (Meesapyodsuk D et al., Metab Engineer 2018, 49, 192).
The increased concentration with age of pentaenoic acid (22:5n-6) or osbond acid was observed for the first time in rat testes, it suggests that lipids may have an essential role in the maturation of the testis (Kirschman JC et al., Arch Biochem Biophys 1961, 93, 297). Later, the biosynthesis of 24:4n-6 and 24:5n-6 were described in rat testes (Bridges RB et al., J Biol Chem 1970, 245, 46). Whereas human spermatozoa contain predominantly di-, tri- and tetraenoic fatty acids with up to 32 carbon atoms, boar, ram and bull spermatozoa contain pentaenoic and/or hexaenoic acids with up to 34 carbon atoms (Poulos A et al., Biochem J 1986, 240, 891). Osbond acid is formed by the stepwise elongation and desaturation of arachidonic acid (20:4 n-6) to the 24 carbon PUFA, 24:6 n-6, and its retroconversion into n-6 DPA.
Several other fatty acids were described in mammals (Poulos A, Lipids 1995, 30, 1) and n-6 fatty acids with up to 6 double bonds and 34 carbon atoms have been determined in sphingomyelin and ceramides extracted from the head of mammalian spermatozoa (Bull, ram) (Furland NE et al., J Biol Chem 2007, 282, 18141 and 18151). Most of these very-long chain fatty acids have an even number of carbon atoms but odd-chains also occurred in lower amounts. The mostly represented fatty acids are 27:4 and 29:4n-6.
Polyenoic n-6 fatty acids with carbon chain lengths from 26 to 38 have been detected in abnormal amounts in brain of patients with the rare inherited disorder, Zellweger syndrome (Sharp P et al., Biochem J 1987, 248, 61). They probably derived by chain elongation of shorter-chain n-6 fatty acids and accumulate because of a lack of a specific coenzyme A synthetase, the first enzyme in the beta-oxidation pathway.
An uncommon (n-6) fatty acid was discovered in retina, c14:2 (n-6), acylating a NH2 terminus of a retinal protein, recoverin, involved in the regulation of the photoreception mechanism (Dizhoor AM et al., J Biol Chem 1992, 267, 16033).
The very long-chain (n-6) fatty acid 34:9 (n-6) has been identified in the freshwater crustacean species Bathynella natans living in caves of central Europe (Rezanka T et al., Tetrahedron 2004, 60, 4261). To date, this compound may be considered as the most unsaturated fatty acid discovered in a living structure.
The 28:7n-6 fatty acid and other very long-chain polyunsaturated fatty acids had been found in fish oil, and these had probably been derived from the diet (Rezanka T, J Chromatogr 1990, 513, 344). Dietary fish oil enriched in very-long-chain polyunsaturated fatty acid has been shown to reduce cardiometabolic risk factors and improves retinal function (Zhi-Hong Yang et al., iScience 2023 Nov 7;26(12):108411).
The identification of 28:7n-6 in several marine dinoflagellates support that hypothesis (Mansour MP et al., Phytochemistry 1999, 50, 541). That very long-chain highly unsaturated fatty acid was shown to be associated with phospholipids, and not with glycolipids (Leblond JD et al., J Phycol 2000, 36, 1103).
Several very long-chain n-6 fatty acids have been isolated from a dinoflagellate Amphidinium carterae, they had 22 to 36 carbon atoms and 3 to 7 double bonds (Rezanka T et al., Phytochemistry 2008, 69, 2391).
Metabolism and function of very-long-chain polyunsaturated fatty acids (>C24) in mammals have been reviewed (Murray M et al., AOCS, lipid library July 23, 2019).
![]()
N-3 FATTY ACIDS
A review presents a broad and relatively complete cross-section of knowledge about n-3 monounsaturated fatty acids, polyunsaturates, an outline of their modifications and the interactions between food fatty acids intake are discussed (Cholewski M et al., Nutrients 2018, 10, 1662).
An interesting review of the relationships between biochemistry, nutritional and epigenetic properties of polyunsaturated fatty acids may be consulted (Benatti P et al., J Am College Nutr 2004, 23, 281).
It was recognized as a separate fatty acid in 1887 (Hazura K, Monatsh 1887, 8, 158) and its structure was elucidated in 1909 (Erdmann E et al., Ber 1909, 42, 1334) while it was synthesized only forty years later (Raphael RA et al.,J Chem Soc 1950, 2100). Linolenic acid is the major fatty acid of plant leaves, stems and roots and is the precursor of the (n-3) series which is essential in fish and probably in other animals. The major sources for human food are soybean (4-10%), canola (7-12%) and walnut (9-15%). Other possible sources of n-3 rich oils are hemp (Cannabis sativa) (18-22%), linseed (45-70%), sacha inchi (Plukenetia volubilis) (45-53%), fig (Ficus carina) (38-44%), camelina (31%), chia (60%), black currant (12-14%), wheat germ (4-10%) and perilla (Perilla frutescens) (> 60%).
The question of the possibility of these plant fatty acids to be the precursors of the long-chain n-3 compounds (EPA and DHA) in transgenic plants has been examined (Napier JA et al., Biochimie 2004, 86, 785, Williams CM et al., Proc Nutr Soc 2006, 65, 42). Recent progress demonstrates the feasibility of using transgenic plants to synthesize long-cain polyunsaturated fatty acids.
Besides Linus, the plant Lallemantia iberica (Lamiaceae), originated from the Caucasus and Middle East regions produces a seed oil rich in linolenic acid (67-74%). This plant, known commonly as dragon’s head, is a potential source of n-3 fatty acid and successfully cultivated in some central and southern European countries (Zlatanov M et al., JAOCS 2012, 89, 1393). The seeds contain the edible oil known as lallemantia oil which has been discovered at a number of archeological sites in northern Greece, dating from the Bronze Age. Chia seeds are tiny black or white seeds which are formed by a plant named ‘chia’ (Salvia hispanica, Lamiaceae) cultivated in Central America. These seeds are one of the highest plant based sources of linolenic acid (approximately 25 to 40 percent of the chia seed is composed of oil and 60 percent of the oil is made of linolenic acid, n-6:n-3 ratio of 1:3).
There is increasing evidence for health benefits of plant-based diets based on the presence of linolenic acid in their major food sources (i.e., walnuts and flaxseed). Meta-analyses of observational studies have shown that increasing dietary linolenic acid is associated with a 10% lower risk of total cardiovascular disease and a 20% reduced risk of fatal coronary heart disease (review in Sala-Vila A et al., Adv Nutr, 2022, 13, 1584). Its role in cognition is in the early stages but shows promising evidence of counteracting cognitive impairment. There is some evidence of benefits of linolenic acid at high intakes (>2 g/d; 0.6–1% of energy). It must be emphasized that several meta-analyses have assessed the importance of the ratio of n-6 FAs and n-3 FAs on the incidence of cardiovascular diseases (Cao M et al., Food Biosci 2024, 59, 104066).
Stearidonic acid occurs also in microorganisms a a minor component but was found at high level (8% of total lipids) in the phosphatidylcholine fraction of various mutants of Mortierella alpina (Jareonkitmongkol S et al., Appl Environ Microbiol 1992, 58, 2196).
Canola seeds have been genetically remodeled to accumulate stearidonic acid, thus facilitating increased compliance with the recommended dietary intake of n-3 fatty acids (Ursin VM, J Nutr 2003, 133, 4271). Similar productions were obtained from soybean transfected with D6 desaturase from borage. This approach was also explored in the production of n-3 enriched linseed oil (Ruiz-López N et al., Plant Biotechnol J 2009, 7, 704).
A review of the efforts focused on the production of n-3 long-chain fatty acids in transgenic plants has been released (Napier JA, Eur J Lipid Sci Technol 2006, 108, 965). A review on stearidonic acid as a potential alternative for marine oil fatty acids may be consulted (Coupland K, Lipid Technol 2008, 20, 152). Review of the possible health benefits of dietary stearidonic acid produced by algae have been written by Whelan J (J Nutr 2009, 139, 5) and Lenihan-Geels G (Nutrients 2013, 5, 1301).
Quarterly news about all aspects of these fatty acids may be found in PUFA Newsletter.
A review of the structural roles of EPA and DHA in cellular membranes in bacteria as well as in multicellular organisms has been established by Valentine RC et al. (Prog Lipid Res 2004, 43, 383). A review focusings on the sources, production, and biological functions of DHA and providing prospective trends may be consulted (Li Q et al. Food Chem 2019, 301, 125286). Distribution – Evolution
They are found in unicellular marine algae which can enter the food chain and are then important nutrients for the health of many animals, including humans. They are also found in brown macroalgae, in moss cells and in many animal tissues (mainly in nervous tissues). DHA is the most abundant fatty acid in the vertebrate brain. Several studies have shown that DHA is itself necessary to support optimal function of the brain and retina (Mitchell DC et al. Biochem Soc Trans 1998, 26, 365).
Based on several data, the unique importance of DHA and its irreplaceability in neural and retinal tissues has been postulated. A new hypothesis provided experimental evidence to support a previously unrecognized key role for DHA in the process of photo-transduction in the retina (Crawford MA et al., Entropy 2023, 25, 1520). They explained that the quantum of energy from the photon-induced cis–trans isomerization of retinal is absorbed by a DHA π-electron and that this hyperpolarization extracts the energized electron, and transmits its information to the brain, conserving the fidelity of the original wavelength. This hypothesis provides a clear rationale to support the observations of the extraordinary high density of DHA in retina and the presence of very long-chain omega-3 PUFAs surrounding the opsins, which serve to absorb the energy.
A review has shown that DHA has only been found in marine mollusks and crustaceans (Fock E et al., Comp Biochem Physiol Part B 2025, 275, 111023). A gradual decrease in the DHA content until its disappearance can be observed in the brain lipids of the series marine-freshwater-terrestrial crustaceans and marine-terrestrial mollusks, suggesting that the transition to the land lifestyle in the evolution of invertebrates, but not vertebrates, was accompanied by a loss of DHA. Furthermore, DHA was not found in insects, either terrestrial or aquatic, or in nematodes. It was also shown that the nervous and visual systems of various DHA-free invertebrates can be highly enriched in 18:3ω3 or 20:5ω3.Before 1977, most of the literature indicates that bacteria do not contain PUFAs with more than two double bonds. In 1977, for the first time, a high proportion of 20:5 n-3 (EPA) was reported in a marine bacterium (Johns RB et al., 1977). Later, a study on 11 piezophilic bacteria (from 1200 to 10 476 m of sea depth) revealed they produced EPA (20:5n-3) and DHA (22:6n-3) in increasing proportion of total fatty acids when pressure increased (DeLong EF et al., Appl Environ Microbiol 1986, 51, 730). The presence of PUFAs in these bacteria most likely represents an adaptation to low temperature rather than to high pressure.
Biosynthesis:
It is well established that DHA can be biosynthesized from linolenic acid (18:3n-3). However, the last desaturase step (D4-desaturase) has been identified only in microalgae (Pereira SL et al., Biochem J 2004, 384, 357). Certain algae produce EPA and DHA as part of normal metabolism. Cultured under specific and tightly controlled conditions, these algae produced commercially oils which are used in infant formula, foods, beverages, and a variety of supplements (Kuratko CN et al., Eur J Lipid Sci Technol 2013, 115, 965). An indigenous marine diatom Odontella aurita OAOSH22 from the east coast of Korea has been isolated and shown to exhibit significant potential as a valuable source of EPA for various bio-industrial applications (An SM et al., Mar Drugs 2023, 21, 563). More interestingly, an alternative pathway for DHA biosynthesis (the anaerobic polyketide synthase pathway) was also reported recently to occur in microorganisms including bacteria and some eukaryotes including Schizochytrium (Heteroconta, Thraustochytriidae) (Metz JG et al., Science 2001, 293, 290). Three strains of thraustochytrids were identified from British North Sea region as promising organisms for the production of DHA in the context of possible future industrial exploitation (Marchan LF et al., J Appl Phycol 2017, 29, 2831).
With the increased demand for EPA and DHA, DuPont has developed a clean and sustainable source of the omega-3 fatty acid EPA through fermentation using metabolically engineered strains of Yarrowia lipolytica, a yeast that can use unusual carbon sources, such as hydrocarbons. A review, was focusing on this technology for EPA production (Xie D et al., Appl Microbiol Biotechnol 2015, 99, 1599).
Earlier, evidence was reported that aerobic and anaerobic pathways of fatty acid biosynthesis could operate within a single species, the bacterium Pseudomonas (Wada M et al., J Bacteriol 1989, 171, 4267). The relative simplicity of this polyketide synthase-like system makes it attractive in terms of transgenic production of polyunsaturated fatty acids in plants (Napier JA, Trends Plant Sci 2002, 7, 51). Similar results have been described with thraustochytrids from Icelandic waters (Stefánsson MÖ et al., Mar Drugs 2019, 17(8). pii: E449). The different pathways involved in fatty acid synthesis has been reviewed (Morabito C et al., Prog Lipid Res 2019, 76, 101007).
In mammals, 22:5 n-3 is elongated to 24:5 n-3 followed by desaturation by a Δ6-desaturase to 24:6 n-3 (nisinic acid). This fatty acid is further transferred to peroxisomes and converted to DHA by ß-oxidation (Sprecher H, Biochim Biophys Acta 2000, 1486, 219). A deficiency in this enzymatic step in patients with the Zellweger syndrome is accompanied with a profound DHA deficiency in all tissues, including the brain and retina (Martinez M, Brain Res 1992, 583, 171). It was observed that DHA biosynthesis by Δ4-desaturation could be enhanced in the human species by transfecting the enzyme in human lymphocytes, and it should be determined if that approach could normalize the DHA levels in cells from Zellweger patients (Martinez M et al., Lipids in Health and Didease 2010, 9:98). An update to the DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion in mammals has been released (Adam H et al., Prog Lipid Res 2019, 76, 101008).
The liver and the brain astrocytes are considered to be the main sites for biosynthesis of DHA. An omega-3 index may be calculated to appreciate the n-3 status of an animal. This index was defined as the sum of EPA and DHA expressed as a molar percentage of the total fatty acids, classically in red blood cell membranes (Harris WS et al., Prev Med 2004, 39, 212).
Sources of EPA and DHA:
The vast majority of EPA and DHA comes from marine sources including fatty or oily fish (body or liver oils), marine crustaceans such as krill and Calanus. Among animals, the main sources for human nutrition are :
Anchovy oil (primary oil provided in omega-3 supplements from a number of different fisheries throughout the world).
Cod liver oil (one of the first commercially sold omega-3 dietary supplements in the modern marketplace).Pollock oil
Pollock oil (mostly extracted from the livers of pollock caught in Alaska).
Salmon oil (salmon by-products are cold-pressed to make oils).Tuna oil
Tuna oil (made from the by-products of skipjack and yellowfin tuna, often used to fortify infant formulas because of their high DHA content).
Fish roe oils (made from the roe of different fish such as herring).
Krill oil (tiny crustaceans from the waters of the Antarctic — provide a phospholipid source of EPA and DHA).
Calanus oil (a growing market for EPA and DHA supplements made from Calanus, a small crustacean from the Arctic).
Squid oil (made from the by-products of the squid meat production industry, which is relatively high in DHA).
Green-shelled mussel oil (New Zealand’s green-shelled mussels contain EPA and DHA, used in supplements.
One of the main sources of n-3 fatty acids is fish oil but the market prices of that product are increasing significantly. This has prompted a significant amount of research on the use of single-cell oils as a source of n-3 fatty acids. Some of the microorganisms (phototrophic or heterotrophic) reported to produce edible oil that contains omega-3 fatty acids are from the genus Schizochytrium, Thraustochytrium and Ulkenia. An overview of advances in the production of single cell oils rich in n-3 fatty acids may be consulted (Armanta RE et al., JAOCS 2013, 90, 167). The need for a sustainable replacement for diminishing fish stocks as source of EPA has driven many efforts towards the search of its possible synthesis in transgenic plants (Sayanova OV et al., Phytochemistry 2004, 65, 147).
Seeds from Agathis robusta, an Australian primitive gymnosperm (Araucariaceae), were shown to contain small amounts of EPA (together with arachidonic acid), probably deriving from stearidonic acid (Wolff RL et al. Lipids 1999, 34, 1083).
EPA has been identified as a significant component in several fungal and algal oils but none of these has been exploited commercially. The levels of EPA and DHA in bacteria are not as high as in some species of fungi, these fatty acids being stored in fungi oils (triacylglycerols) while there are stored in bacterial membranes. In the fungi Mortierella, 15 % EPA may be found in triacylglycerols (up to 40% of the fungal dry weight).
The production of EPA and DHA by microorganisms (fungi, microalgae) has been extensively reviewed by Ratledge C (Structured and modified lipids, Gunstone FD Ed, M Dekker, NY 2001, p.351), Russell NJ et al. (Microbiology 1999, 145, 767) and Harwood JL et al. (Biochimie 2009, 91, 679).
The Martek company has produced a single-cell oil containing about 40% of DHA from the heterotrophic microalga Crypthecodinium cohnii, this product being used in many food systems such as milk (Kyle DJ, Lipid Technol News 1997, 3, 100). EPA is currently produced from cultures of various microalgae like Phaeodactylum and Monodus. An extensive overview of process investigations related to microbial and microalgae productions of DHA and EPA may bee consulted (Owen PW et al., Proc Biochem 2005, 40, 3627).
The production of EPA and DHA in transgenic plants which might lead to a sustained source of these fatty acids for use in human and animal food was reviewed by Domergue F et al. (Trends Plant Sci 2005, 10, 113). A review of the efforts focused on the production of n-3 long-chain fatty acids in transgenic plants has been released (Napier JA, Eur J Lipid Sci Technol 2006, 108, 965). Among the various strategies which have been considered for the production of n-3 polyunsaturated fatty acids from plants, one of the most promising seems to be the use of transgenic plants ‘‘reverse-engineered” to produce these so-called fish oils (Venegas-Caleron M et al., Prog Lipid Res 2010, 49, 108). A documented review may also be consulted (Sayanova O et al., Prost Leukotr Essent Fatty acids 2011, 85, 253). A new DHA-producing Camelina sativa transformed with a suite of microalgal llong-chain n-3 genes sourced from several target microalgal strains has been reported (Petrie JR et al., PLoS One 2014, 9, e85061). The synthesis of DHA occurs by the elongation and desaturation of C18 PUFA through EPA and not by retro-conversion of 24:6 n-3.
An update on sustainable sources of n-3 oils may be consulted (Nichols PD et al., Nutrients 2010, 2, 572).
Health benefits:
EPA and DHA are important components of fish oil triacylglycerols and their health benefits are claimed to be diverse and orientated against many human disorders (see the site “Fats of Life“). In commonly eaten fish, trout and salmon contained relatively high concentrations of n-3 fatty acids and low n-6/n-3 ratios (Weaver KL et al., J Am Diet Assoc 2008, 108, 1178). While the beneficial effects of DHA have been observed in many unrelated afflictions, its mode of action remains unresolved. The involvement of its oxidation products (hydroxylated derivatives, resolvins, F4 isoprostanes, F4 isofuranes) has been suggested (Siddiqui RA et al., Chem Phys Lipids 2008, 153, 47). A brief review of the current and official acceptance of health benefits from long-chain n-3 fatty acids has been released by Ackman RG (Inform 2004, 15, 550).
Nervous system:
It was suggested that the evolution of the large human brain depended on a rich source of DHA from vegetal or animal food (Crawford MA et al., OCL 2004, 11, 30).
DHA was shown to be directly involved in neuronal survival through phosphatidylserine synthesis and cellular signaling (Akbar M et al., PNAS 2005, 102, 10858). The multiple effects of DHA on eye photoreceptors (protection by delaying apoptosis, promotion of opsin expression) suggest that, in addition to its structural role, DHA might be one of the trophic factors required by these cells (Politi L et al., Lipids 2001, 36, 927). A meta-analysis study has shown that increasing dietary intake of EPA and DHA cuts the risk for age-related macular degeneration (AMD) (Lee Y et al., Front Nutr 2024, 11, 1403987). This could further support the idea that consuming foods rich in long-chain n-3 fatty acids and reducing consumption of foods rich in trans fatty acids) may prevent AMD.
It has been demonstrated that from a certain level of chronic DHA (together with free estradiol deficiency), a permanent imbalance is established with a disruption of glucose intake and cerebral metabolism. This depletion, particularly in DHA, is associated with pathologies of the central nervous system encompassing neurodegeneration and cognitive defects. It is an aggravating factor in certain pediatric neuropathologies such as hyperactivity, learning difficulties, mental retardation, epilepsy and autism (review in : Majou D et al., OC 2023, 30, 22). DHA was shown to increase also the action of brain-derived neurotrophic factor (Majou D et al., OC 2024, 31, 1). The findings of a large study have contributed to the development of precise nutritional intervention with n-3 fatty acids for different populations concerning the improvement of healthy longevity (Wu D et al., Front Nutr 2024, 11). The effect of n-3 fatty acid supplementation on cognitive defects appears to be influenced by baseline homocysteine level, suggesting that adequate B vitamin status is required to obtain beneficial effects of n-3 fatty acids on cognition (Jernerén F et al., J Alzheimers Dis 2019, 69, 189).
The oxidation of DHA may lead to the formation of neuroprostanes, isoprostane-like compounds which are uniquely enriched in the brain.
An extensive review of the known benefits of EPA and DHA may be consulted (Richard C et al., Adv Nutrition 2024, 15, 100161). A theory concerning the role of DHA in brain function at the molecular state in membranes may be read to have an overview of the role of that fatty acid in neural signaling (Crawford MA et al., OCL 2018, 25, A403).
A systematic review of the literature on the effects of n-3 fatty acids on cognitive function in aging people may be consulted (Issa AM et al., Dement Geriatr Cogn Disord 2006, 21, 88). Authors concluded that the available data (497 studies) are insufficient to draw strong conclusions about the effects of n-3 fatty acids on cognitive function in normal aging or on the incidence of treatment of dementia. A more recent study (Zutphen Elderly Study) has concluded that fish consumers (210 participants aged 70-89, 20 to 400 mg EPA + DHA per day) had less 5-year subsequent cognitive decline than did non-consumers (Van Gelder BM et al., Am J Clin Nutr 2007, 85, 1142). An update on omega-3 polyunsaturated fatty acids (n-3 PUFA) in preventing cognitive decline and dementia may be consulted (Welty FK, Curr Opinion Lipidol 2023, 34, 12). The impact of n-3 fatty acids on the use of brain glucose suggests that dietary intake of n-3 fatty acids can help to reduce the cognitive deficits in the elderly and possibly symptomatic cerebral metabolic alterations in Alzheimer disease (Harbeby E et al., OCL 2012, 19/4, 238).
The interaction between DHA and its metabolites (neuroprotectins) and its involvement in the pathogenic processes characteristic of aging and neurodegenerative diseases have been reviewed (Lukiw WJ et al., J Nutr 2008, 138, 2510).
Findings suggest that EPA and DHA and inflammatory factors (TNF-α and CRP) may predict the occurrence and the severity of cognitive impairment among elderly depressed patients (Gao J et al., J Affect Disord 2023, S0165-0327(23)01350-2).
Cardiovascular system:
The differential effects of EPA, DPA (22:5n-3) and DHA on cardio-metabolic risk factors in high-fat diet fed mice have been explored (Guo XF et al., Prostaglandins Leukot Essent Fatty Acids 2017, pii: S0952-3278(17)30120-5). Additionally,the interest in encapsulated omega-3 formulations has been proposed for cardiovascular disease treatment (Gill R et al., Mar Drugs 2024, 22(6), 256).
In 2004, the Omega-3 Index – defined as the sum of EPA + DHA in % of total fatty acids (FA) in red blood cells (RBC) – was first proposed as a risk factor for death from coronary heart disease (Harris WS et al., Prev Med 2004, 39, 212). At that time, and based on data then available, an Omega-3 Index of >8% was proposed as a healthy or optimal target for reducing risk. Omega-3 Index values of 4%–8% were considered “intermediate”, and <4% was associated with the highest risk. The update of the original map reflected a more current worldwide picture of n-3 status in populations (Schuchardt JP et al., Prog Lipid Res 2024, 95, 101286). It was found that the Omega-3 Index in most countries was low to very low.
Basic mechanisms behind the omega-3 effects on cardiovascular disease have been also reviewed (Massaro M et al., Prost Leukotr Essent Fatty Acids 2008, 79, 109).
Inflammation:
It is now largely accepted that EPA and DHA are able to partly inhibit a number of aspects of inflammation including leukocyte chemotaxis, adhesion molecule expression and leukocyte–endothelial adhesive interactions, the production of prostaglandins and leukotrienes from the n-6 fatty acid arachidonic acid, the production of inflammatory cytokines, and T-helper 1 lymphocyte reactivity. The known mechanisms and clinical relevance of these properties have been reviewed (Calder PC, Biochim Biophys Acta 2015, 1851, 469).
It has been shown than ratios 1:1 and 2:1 EPA:DHA evidenced a noteworthy healthy effect generating a less oxidative environment and modulating LOX and COX activities toward a decrease in the production of proinflammatory eicosanoids derived from arachidonic acid and oxidative stress biomarkers from EPA and DHA (Dasilva G et al., J Nutr Biochem 2015, 26, 1385). These results in rat support the anti-inflammatory and antioxidative role of fish oils and, particularly, the effect of adequate proportions EPA:DHA.
Krill oil and Its bioactive components as a potential therapy for inflammatory bowel disease has been reviewed (Liu Y et al., Biomolecules 2024, 14(4), 447).
The potential effects of various dietary EPA and DHA ratios (1:1, 2:1, and 1:2, respectively) on protein redox states from plasma, kidney, skeletal muscle, and liver were demonstrated in rats (Mendez L et al., Free Rad Biol Med 2013, 55, 8). A supplementation with an equal EPA:DHA ratio (1:1) showed the lowest oxidation score for plasma albumin, followed in increasing order of carbonylation by 1:2 < 2:1. The results confirmed the efficiency of fish PUFA supplementation at increasing the levels of EPA and DHA in tissues and at protecting proteins against in vivo oxidation.
After showing an effect of DHA on cellular antioxidant capacity and mitochondrial functions, it has been suggested that DHA’s antioxidant activity can be attributed to its enhancement of mitochondrial functions and biogenesis (Li G et al., J Agric Food Chem 2021, 69, 1647).
Reproduction:
A study using delta-6 desaturase knockout mice demonstrated that DHA supplementation restored spermatogenesis and fertility in the absence of 22:5n-6 and low 20:4n-6 in testis, while dietary 20:4n-6 was much less effective (Roqueta-Rivera M et al., J Lipid Res 2010, 51, 360). Furthermore, these mice developed intestinal ulcers and severe dermatitis despite an adequate supply of linoleic acid and gamma-linolenic acid from diet. It has been also demonstrated that acyl-CoA synthetase isoform 6 is an important driving force for germ cell DHA enrichment and is required for normal spermatogenesis and male fertility in mouse (Hale BJ et al., J Biol Chem 2019, 294, 14394).
Cancer:
A systematic survey of 38 studies revealed that there was no significant association between n-3 fatty acid consumption and cancer incidence (MacLean CH et al., JAMA 2006, 295, 403). Despite these results, the putative mechanisms whereby marine n-3 fatty acids may modulate the carcinogenic process in some experiments were examined in another review (Larsson SC et al., Am J Clin Nutr 2004, 79, 935).
Marine fish oil is a good dietary source of the omega-3 fatty acids docosahexaenoic acid and eicosapentaenoic acid, which have been shown to have anti-cancer properties and could have several implications for mutitergeted cancer therapy (D’Eliseo D et al., J. Clin. Med. 2016, 5, 15), including potential applications in the management of gastrointestinal cancer (Eltweri AM et al., Clin. Nutr. 2017, 36, 65).
An extensive review of the membrane properties of DHA may be consulted for further information (Stillwell W et al., Chem Phys Lipids 2003, 126, 1).
EPA contained in galactosyl diglycerides and phospholipids of marine diatoms was shown to be the source of a short-chain aldehyde, heptadienal (7:2 n-3), which participates to deleterious effects on zooplankton crustaceans (d’Hippolito G et al., Biochim Biophys Acta 2004, 1686, 100).
DHA was shown to be oxidized, as arachidonic acid, into isoprostane-like compounds (neuroprostanes) which seem to be of great value to appreciate oxidative injury to the neural tissues and to generate hydroxylated derivatives (docosatrienes) which are potent in preventing inflammation (resolvins, neuroprotectins).
Uncommon n-3 fatty acids
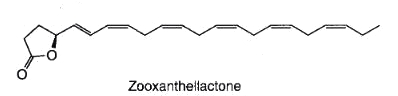
A novel fatty acid derivative named zooxanthellactone was isolated from several strains of symbiotic microalgae, dinoflagellates of the genus Symbiodinium (Onodera K. et al., Biosci Biotechnol Biochem 2004, 68, 848).
Two uncommon (n-3) fatty acids were described in cultures of fibroblasts, 14:3 n-3 and 16:4 n-3, as metabolites produced by normal conversion of 20:5(n-3) in peroxisomes (Williard DE et al., J Lipid Res 1998, 39, 978). 16:4(n-3) was also identified in the extract of the sponge Callyspongia sp found off the coast of New South Wales, Australia (Urban S et al., Lipids, 1997, 32, 675). The 16:4 n-3 is produced in mesenchymal stem cells treated with chemotherapeutic agents and was shown to be a potent mediator of resistance to chemotherapy, its production may be a target to enhance chemotherapy efficacy in patients (Roodhart JML et al., Cancer Cell 2011, 20, 370).
It has been shown that mesenchymal stem cells activated by platinum-based chemotherapy secrete 16:4 n-3, together with 12-oxo-5,8,10-heptadecatrienoic acid, which in minute quantities confer resistance to multiple types of chemotherapy (Roodhart JML et al., Cancer Cell 2011, 20, 370). Later, it has been demonstrated that 16:4(n-3) exerts its effect by activating splenic F4/80+/CD11b macrophages, which results in the production of chemoprotective lysophosphatidylcholines (Houthuijzen JM et al., FASEB J 2017, 31, 2195). These results suggest that it might be possible to limit the development of chemotherapy resistance.
An unusual n-3 fatty acid, anteiso-heptadeca-9,13-dienoic acid (17:2 n-3), was discovered in lipids extracted from the cell membrane phospholipids of Chryseobacterium frigidisoli PB4T, a psychrotolerant, non-motile, gram-negative, aerobic bacterium isolated from ice free permafrost deposits in continental Antarctica (Mangelsdorf K et al., Org geochem 2017, 106, 68). The presence of that fatty acid suggests its importance for cell membrane adaptation of C. frigidisoli with respect to low ambient temperature conditions.
An unusual n-3 polyunsaturated fatty acid, 18:5 n-3, was isolated from a raphidophyte alga, Heterosigma akashiwo (Bell MV et al., Phytochemistry, 1997, 45, 303). The high abundance of 18:5n-3 in some dinoflagellates, prasinophytes, haptophytes and raphidophytes (see Bell et al., 1997 for references) may be due to a chain-shortening mechanism from 20:5n-3 as demonstrated for 22:6n-3 formed by chain-shortening from 24:6n-3. That fatty acid has in the past be considered one of the more valuable fatty acids characterizing dinoflagellates among algae. Now, other long-chain fatty acids, 28:7n-6 and 28:8n-3, are considered better chemotaxonomic compounds for that algal class. It is noteworthy that 18:5n-3, as 18:4n-3, were shown to be primarily associated with glycolipids in dinoflagellates (Leblond JD et al., J Phycol 2000, 36, 1103).
The methylated derivative of octadecapentaenoic acid was also isolated from the marine dinoflagellate Karenia mikimotoi and was shown to have a potent anti-inflammatory activity (Leutou AS et al., Mar Drugs 2020, 18, 138).
In some plants (spinach, tobacco, fresh-water algae), an uncommon trienoic fatty acid 16:3 n-3 (7,10,13-hexadecatrienoic acid), known also as roughanic acid, is found and was the base of a division of plants into the so-called “16:3-plants” (prokaryotic-like) and “18:3-plants”. This fatty acid is mainly located in glycolipids of the chloroplast membranes. It has been identified in linolenic rich lipid extracts from various plants: Chinese cabage, kale, brussels sprouts, cauliflower, and parsley (Nasirullah et al., Eur. J. Lipid Sci Technol 1984, 86, 264). It has been also detected and quantified in lipid extracts of some conifer species (preferentially concentrated in the mono-galactosyl diglycerides) (Jamieson GR et al., Phytochemistry 1972, 11, 269).
Along with some angiosperms, it was shown that the occurrence of 16:3 n-3 is characteristic of prokaryotic organisms such as cyanobacteria, as well as of microalgae, mosses, ferns, conifers, and other eucaryotic plant groups of a lower taxonomic position. Several dioxygenation products of this fatty acid have been detected in seedlings of some plant species (Osipova EV et al., Biochemistry. Biokhimiia, 2010, 75, 708).
Docosatrienoic acid (22:3 n-3) is a long chain polyunsaturated fatty acid that has been recently shown to possess strong anti-inflammatory and antitumor properties (Chen Y et al., Nutr Cancer, 2021, 73, 1697). Improved production of this fatty acid in Brassica carinata crop using biotechnology provides a real commercial opportunity for high value agriculture products for nutraceutical uses (Meesapyodsuk D et al., Metab Engineer 2018, 49, 192)
Very long chain (n-3) fatty acids were described in the blubber of seals (Phoca hispida) from northern fresh or seawater. Thus, 23:5, 24:3, 24:4, 24:5, 24:6, 26:5, 26:6 and 28:7 were found at a concentration not exceeding 0.2% (Kakela R et al., Lipids 1995, 30, 725). In Ophiuroidea (Brittle star) 24:6n-3 (nisinic acid) has been observed for the first time at concentrations of 3-15% in total fatty acids and particularly in some phospholipids extracted from Cridoidea and Ophiuroidea (Takagi T et al., Lipids 1986, 21, 430). It has been proposed that 24:6n-3 is a biomarker for brittle stars (Ophiuroidae) (Mansour MP et al., Biochem Syst Ecol 2005, 33, 659).
It is also an especially important component in lipids of the Cnidaria species such as jellyfish (Nichols PD et al., Lipids 2003, 38, 1207) and soft corals (Imbs A et al., Comp Biochem Physiol B 2007, 148, 314); as a minor component, it has been found in some fish species (Baltic herring, flathead flounder, and eel). This fatty acid was also characterized in several other marine animals (cnidarian, echinoderm) (Barnathan G, OCL 2010, 17, 238-250).
Purified 24:6n-3 from the brittle star Ophiura sarsi was found able to inhibit the antigen-stimulated production of leukotriene-related compounds such as LTB4, LTC4, and 5-hydroxyeicosatetraenoic acid, as well as other n-3 PUFAs, 20:5n-3 and 22:6n-3, in a mouse mast cell line (Ishihara K et al., Lipids 1998, 33, 1107). It also could reduce the histamine content in MC/9 cells. The patterns of the effects of 24:6n-3 on the eicosanoid synthesis and histamine content are more similar to those of 22:6n-3 than 20:5n-3. Thus, anti-inflammatory and antiallergic activities of 24:6n-3 similar to those of 22:6n-3 can be expected.
A comparison of the effect of n-3 PUFAs (20:5n-3, 22:5n-3, 22:6n-3, and 24:6n-3) on lipid metabolism of HepG2 cells has shown that 24:6n-3 has the strongest ability to suppress the synthesis of triacylglycerols and cholesteryl ester among these n-3 fatty acids (Nagao K et al., J Oleo Sci 2014, 63, 979).
In several deep water species of Ophiuroidea C26 acids have been characterized as 5,8,11,14,17,20,23-hexacosaheptaenoic [26:7(n-3)]; 8,11,14,17,20,23-hexacosahexaenoic [26:6(n-3)] and 11,14,17,20,23-hexacosapentaenoic [26:5(n-3)]. The concentrations of these acids varied from 0.3 to 4.5 mol% of the total fatty acids. The main component of these C26 fatty acids is hexacosaheptaenoic acid 26:7(n-3) (Svetashev V., et al., Lipids 2015, 50, 691).
Ram and bull spermatozoa are particularly rich in the longer chain polyenoic fatty acids, those having 30 to 34 carbon atoms and 6 double bonds (Poulos A, Lipids 1995, 30, 1).
The presence of the most unsaturated (n-3) fatty acid (28:8 n-3) was detected in oil derived from the dinoflagellate Crypthecodinium cohnii. As 22:6 n-3, it contains the maximal number of methylene-interrupted double bonds in the fatty acid chain with also a -CH2-CH2– group at both ends of the molecule (Van Pelt CK et al., J Lipid Res 1999, 40, 1501). That very long-chain highly unsaturated fatty acid was shown to be associated, as for 28:7n-6, with phospholipids, and not with glycolipids (Leblond JD et al., J Phycol 2000, 36, 1103). It was shown to be also present in another dinoflagellate Prorocentrum micans (Mansour MP et al., Phytochemistry 1999, 50, 541). Furthermore, this fatty acid was also detected in a sample of fish oil concentrate.
Several very long-chain n-3 fatty acids have been isolated from a dinoflagellate Amphidinium carterae, they had 22 to 36 carbon atoms and 3 to 7 double bonds (Rezanka T et al., Phytochemistry 2008, 69, 2391).
A new n-3 fatty acid has been described in a red alga of the genus Laurencia : tetracosa-9,12,15,18,21-pentaenoic acid in the ethyl form (Feng MT et al., Chem Nat Compounds 2015, 51, 418). This fatty acid exhibited moderate antifungal activity against Candida glabrata (MIC 80 value of 4 microg/ml and against Cryptococcus neoformans (MIC 80 value of 8 microg/ml). This alga has been proved to be some of the most prolific producers of structurally unusual secondary metabolites in the marine environment.
![]()
Among that series, the best known compound is the trienoic 20:3(n-9) (Mead acid) with the double bonds in positions 5,8 and 11. It was discovered first by Klenk E. (Z Physiol Chem 1952, 291, 104 and 1955, 299, 74) in brain phospholipids but its exact structure has been determined by Mead JF (Mead JF et al., J Biol Chem 1956, 219, 705). It was shown to be present in relatively high amounts in all tissues of animals subjected to long-term deprivation of nutritionally essential (n-6) fatty acids or tofat-free diets (Holman RT, J Nutr 1960, 70, 405). This author had proposed the ratio of 20:3(n-9) to 20:4(n-6) as a measure of essential fatty acid requirement. Recently, it was reported the presence of unusually high levels of this fatty acid in the cartilage of several animal species (birds, mammals, human). Its concentration in phospholipids was about 5% in the growth plate cartilage and 16% in the hyaline cartilage in chicken (Adkisson HD et al., FASEB J 1991, 5, 344). As 20:3(n-9) is markedly concentrated in human fetal cartilage, it has been hypothesized that it can decrease osteoblastic activity and thus may be important for the prevention of calcification in the cartilage (Hamazaki T et al., Lipids 2009, 44, 97). These authors suggest that the presence of this fatty acid in cartilage may be related to its vessel-free status, thus it could be useful for the treatment of disorders with excessive vasculature (Hamazaki T et al., Prost Leukotrienes Essential Fatty acids 2012, 86, 221).
Recent pathological and epidemiological studies on Mead acid raised the possibility of its effects on inflammation, cancer, dermatitis and cystic fibrosis, suggesting it is an endogenous multifunctional PUFA. A review summarized the biosynthesis, presence, metabolism and physiological roles of Mead acid and its relation to various diseases (Kawashima H et al., Lipids Health Dis 2023, 22, 172).
An unusual geometrical isomer of 22:4 n-9 with a trans double bond (cis-4,7,10,trans-13-docosatetraenoic acid) has been identified in phospholipids of the scallop Pecten maximus and may be of endogenous origin (Marty Y et al., J Chromatogr A 1999, 839, 119). Furthermore, it was found particularly concentrated in the plasmalogen and diacyl forms of serine glycerophospholipids. A survey of the distribution of that new fatty acid has shown that it is present in all genus of the Pectininae and of the Aequipecten subgroup (Kraffe E et al., Lipids 2010, 45, 437).
Two unusual n-9 fatty acids have been described in lipids of deep-sea Foraminifera: Δ4,11-18:2 and Δ5,8,11,14-23:4 (Kharlamenko VI et al., Lipids. 2017, 52, 345).
![]()
Several other polyenoic acids are described in marine algae and are used as marker for several microalgae in the marine environment. Thus, 16:2 (n-7), 16:2 (n-4) have been suggested as tracers for diatoms, 16:2 (n-6) and 16:4 (n-3) for Chlorophyceae and 18:5 (n-3) for dinoflagellates (Viso AC et al., Phytochemistry 1993, 34, 1521). The latter was discovered in 1975 (Joseph JD, Lipids 1975, 10, 395) in 11 species of photosynthetic dinoflagellates, some of them being present at a concentration of about 20% (wt/wt) in total lipids (Gymnodinium, Peridinium, Massartia, Prorocentrum).
As various species of phytoplanktonic herbivorous copepods were shown to contain this uncommon fatty acid, it was proposed as a possible tracer in the marine food chain (Mayzaud P et al., Lipids 1976, 11, 858).
Seed oils of Androsace septentrionalis (Primulaceae) (Tsevegsuren N et al., Lipids 2003, 38, 1173) and of several species of Sapindaceae (Spitzer V, Phytochemistry, 1996, 42, 1357) were shown to contain 16:2 n-4 (about 5% of the total fatty acids), it was previously only known in diatoms.
Another unusual fatty acid, 16:3 n-4, was shown to be abundant (about 45%) in galactosyl diglycerides from marine diatoms (d’Hippolito G et al., Biochim Biophys Acta 2004, 1686, 100). It was shown to be the source of a short-chain aldehyde, octadienal (8:2 n-4), which participates to deleterious effects on zooplankton crustaceans.
Other unusual fatty acids have been described from lipids of deep-sea Foraminifera (Kharlamenko VI et al., Lipids. 2017, 52, 345).
A fatty acid, related to the ω2 family, Δ5,8,11,14,17,20–22:6(n‐2) has been described in abyssal Echinodermata (the sea star (Asteroidea) Eremicaster vicinus and the sea urchin (Echinoidea) Kamptosoma abyssale) from the Kuril–Kamchatka Trench and collected at depths of 5210 and 6183 m. That fatty acid amounted 1.60% and 0.33% of total fatty acids, respectively, for the two species. Such unusual fatty acid can be explained by the transfer and modification of fatty acids from consumed foraminifera, which in turn, feed on bacteria (Svetashev V et al., Lipids 2020, 55, 291).
The 16:4n-1 (6,9,12,15-hexadecatetraenoic acid) has been isolated and characterized in fish oil (Li D et al., Chromatographia 2012, 75, 1-6).
Five novel non‐methylene‐Interrupted dienoic and trienoic fatty acids with a terminal double bond were identified in ovaries of the limpet Cellana toreuma as 5,14‐pentadecadienoic acid (15: 2Δ5,14), 5,9,18‐nonadecatrienoic acid (19:3Δ5,9,18), 5,9,20‐heneicosatrienoic acid (21:3Δ5,9,20), 7,13,20‐heneicosatrienoic acid (21:3Δ7,13,20), and 5,9,22‐tricosatrienoic acid (23:3Δ5,9,22) (Kawashima H, Lipids 2020, 55, 285).
Sebaleic acid (18:2 n-10) was found to be the most important diene fatty acid present in human sebum (Nicolaides N et al., Lipids 1969, 4, 79), in human hair and nails (Destaillats F et al., J Chromatogr A 2011, 1218, 9384). This unusual compound is synthesized only in sebaceous cells by elongation and desaturation of sapienic acid (16:1 n-10) and that its importance may be related to the sebaceous gland activity (Stewart ME et al., J Invest Dermatol 1986, 87, 733).
An unusual hexatrienoic acid with a terminal double bond aliphatic chain (Δ9,12,15-16:3) has been described in Sorghum bicolor (Pan Z et al., J Biol Chem 2007, 282, 4326). This fatty acid is used along a definite pathway in the formation of sorgoleone (a lipid quinone) produced by roots which is likely responsible for the inhibition of the germination of other grass weeds.
All polyenoic acids have very low melting points and are highly susceptible to oxidative degradation (peroxidation). UV radiation, high temperature, oxygen, metals and alkaline conditions are efficient to alter these molecules by migration of double bonds which are thus not separated by a methylene unit(conjugated double bonds), peroxidation and fragmentation. In contrast to trans fatty acids, conjugated fatty acids are not formed in higher amounts during industrial hydrogenation. These forms arise in the first stomach of ruminants as intermediates of dietary unsaturated fatty acids during bacterial fermentation. The first step is the isomerization of linoleic acid to mainly c9c11-18:2 catalyzed by the anaerobic Butyrivibrio fibrisolvens.
Odd-chain polyunsaturated fatty acids are present in small amounts in various living organisms. Protists, such as those of the genera Schizochytrium and Thraustochytrium contain these fatty aids in unusually high qualitative (from C19:1 to C19:4 and from C21:1 to C21:6) and quantitative amounts (C21:4ω7 constitutes 7.2 % of total FA) (Chang KJL et al., Phytochemistry, 2011, 72, 1460). Another protist, Khawkinea quartana contains odd-chain fatty acids, e.g., C17:4, C19:5, C21:4, etc. (Řezanka T et al., Lipids, 2015, 50, 811). The filamentous fungus Saprolegnia produced odd-chain fatty acids (C17:2ω5, C19:4ω5, and C19:5ω2) when grown with saturated fatty acids with 13, 15, or 17 carbons (Shirasaka N et al., Biosci Biotechnol Biochem 1995, 59, 1963). Chytrid fungi grown in a complex medium produced odd-chain PUFA (C17:2ω8 or C17:3ω5) in a maximum of approximately 3 % of total FAs (Akinwole PO et al., Lipids, 2014, 49, 933).). The growing demand for improving the nutritional value of food has driven efforts to enhance the production of polyunsaturated fatty acids (PUFAs) through microbial cultivation (Palyzová A et al., Food Chem 2025, 483, 144204).
In insects, a C21 trienoic acid (3,6,9-henicosatriene) and a C21 tetraenoic acid (1,3,6,9-henicosatetraene) have been isolated from an Arctiidae insect, Syntomoides imaon, and displayed sex pheromone activity (Matsuoka K et al., J Chem Ecol 2008, 34, 1437). They can be considered as longer-chain analogs of linolenic acid.
![]()
CONJUGATED DIENES
Positional isomerization can take place in polyunsaturated fatty acids. This transformation is characterized by a shift of isolated double bonds towards a structure in which unsaturated centers are immediately adjacent to each other. It was discovered in 1933 that the formation of conjugated dienes in fats or oils gives rise to an a!sorption peak at 230-235 nm in the ultraviolet region (similarly conjugated trienes a!sorb at 268 nm). In the 1960s, monitoring diene conjugation emerged as a useful technique for the study of lipid oxidation. The conjugation of the double bonds is considered as an intermediate step in polyene acid peroxidation. Thus, an increase in Uv absorption theoretically reflects the formation of primary oxidation products in fats and oils (Shahidi F et al., Food Res Int 1994, 27, 555).
Conjugated linoleic acid (CLA, a collective term used to designate a mixture of positional and geometric isomers of linoleic acid) is the first step in linoleic acid peroxidation. Its determination is currently used as an assay of free radical activity, but recent studies have cast doubt on the specificity of the assay since bacteria are able to induce their formation (Jack CI et al., Clin Chim Acta 1994, 224, 139) without excluding any dietary origin (Briton M et al., Clin Sci 1992, 83, 97-101).
The two double bonds in CLA are primarily in position 9 and 11, and 10 and 12 along the carbon chain. There can also be geometric changes (cis or trans configuration). Thus, at least 8 different CLA isomers of linoleic acid have been identified. Of these isomers, the c9, t11 form is believed to be the most common natural form with biological activity. It can be considered as the principal dietary form, accounting for as much as 85-90% of the total CLA content in dairy products. The name “rumenic acid” has been proposed as a common name for that major CLA isomer found in natural products (Kramer JKG et al., Lipids 1998, 33, 835). Additional potentially active isomers are also being identified and studied (Belury MA, Nutr Rev 1995, 53, 83; Yurawecz MP et al. Lipids 1998, 33, 803).
CLA were first isolated by Pariza in 1983 from ground meat but their structure was determined later (Ha YL et al., Carcinogenesis 1987, 8, 1881). They occur naturally in food and are present at high concentrations in products (milk and cheese) from ruminant animals (cattle, sheep). They are also found to be present in a variety of dairy products (Ha YL et al., J Agr Food Chem 1989, 37, 75; Chin SF et al., J Food comp anal 1992, 5, 185). Meat products contain 3 to 6 mg CLA/g fat in beef, veal, and lamb, 0.6 in pork and 0.9 in chicken. Dairy products contain 1.5 to 30 mg CLA/g fat in milk, butter, cream, yogurt, and cheese.
The profile of the conjugated linoleic acids in human milk has been determined (Luna P et al., Eur J Lipid Sci Technol 2007, 109, 1160). Mean values of total CLA varied from 0.12 to 0.15 % of total fatty acids, rumenic acid representing more than 60 % of total CLA. Although most of the isomers were present in all samples, teir concentgrations varied considerably. Rumenic acid has been detected in human milk (about 4 mg/g fat) (Jensen RG et al., Adv Exp Med Biol 2001, 501, 153).
Vegetable oils contain only 0.2 to 0.7 mg CLA/g. An isomer (10t,12t) is present in Chilopsis linearis seed oil.
It was shown that CLA were mainly localized to the sn-1 and sn-3 positions of triglycerides in lamb adipose tissue when metabolically produced from safflower oil, but they had increased proportions at the sn-2 position of triglycerides when animals received pre-formed CLA in the diet (Paterson LJ et al., Lipids 2002, 37, 605).
CLA were shown to be metabolized as for linoleic acid but it seems that the two main isomers, t10,c12 and c9,t11-CLA, show differences in their metabolism. While c9,t11-CLA seems well metabolized up to 20:4, the t10,c12-CLA is mainly transformed in its c6,t10,12c-18:3 metabolite (Sébédio JL et al., Lipids 2001, 36, 575). Thus, CLA isomers may be viewed as a “new” family of polyunsaturated fatty acids (Banni S et al., Lipids 2004, 39, 1143).
CLA are present in triglycerides, phospholipids and lipoproteins. They are synthesized from linoleic acid through biohydrogenation pathways and enzymatic isomerization by rumen bacteria (see Bauman DE et al.). It was shown that CLA are stable, neither destroyed nor generated by cooking or storage.
Interest in CLA was increased when Ha YL et al. (Ha YL et al., Carcinogenesis 1987, 8, 1881) described its anticarcinogenic properties. Furthermore, they were reported to have antiatherogenic and hypocholesterolemic effects, to induce a relative decrease in body fat levels and to modulate immune responses in several animal models, antioxidant activities were also described. The two isomers, t10,c12 and c9,t11-CLA have different biological activities depending on the experimental system. Both have been shown to exert anticancer and anti-atherogenic activities, but the reduction of body fat seems specific to t10,c12-CLA (Pariza MW et al., Prog Lipid Res 2001, 40, 283). Opposing effects of c9,t11 and t10,c12-CLA were described on blood lipids in healthy humans (Tricon S et al., Am J Clin Nutr 2004, 80, 614). Among all isomers, c9,t11-CLA was shown to be the most potent in inhibiting proliferation of normal and leukemic cells (Lai KL et al., Lipids 2005, 40, 1107).
Since these early discoveries several beneficial effects on health of CLA have been reported, mainly in animal models of human diseases and in cultures of various types of animal and human cells. Other effects on immune function, lipid and eicosanoid metabolism, cytokine and immuno-globulin production and many metabolic processes have also been described. As the majority of studies have been carried out using mixtures of isomers in animal models, these observations need to be substantiated before they can be regarded as fact (Wahle KW et al., Prog Lipid Res 2004, 43, 553). Furthermore, CLA, as other conjugated fatty acids, has antioxidative activity which could be related to their anticarcinogenic and anti-atherosclerotic effects (Yang L et al., J Agric Food Chem 2009, 57, 4212). Preclinical studies, particularly those involving the t10c12 isomer, have shown that CLA can enhance bone mineral density (BMD) by enhancing bone formation and reducing bone resorption, indicating its potential as a therapeutic agent to improve bone health (Hoque K et al., Nutrients 2025, 17, 1395).
For a review on the occurrence and physiological properties of trans fatty acids and on CLA, see: Fritsche J et al. (Fett/Lipid 1998, 100, 190), and a page of the site of Dr Christie. An overview of the literature on the effects of CLA on body composition and plasma lipids in humans was released by Terpstra AHM (Am J Clin Nutr 2004, 79, 352). After hundred of reports have been accumulated, a definite conclusion for CLA safety has not been reached, while they could have beneficial health effects for humans (review in: Benjamin S et al., Nutr Metabol 2009, 6, 36). An insight into their health benefits up to 2009 has been summarized (Benjamin S et al., Nutr Metab 2009, 6, 36). More recently, several reports suggest that CLA have potent anti-carcinogenic, lipid metabolism regulation, anti-inflammatory, anti-obese and antioxidant activities (review by: Yuang GF et al., Food Funct., 2014, 5, 1360). The numerous effects of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance have been more recently reviewed (Putera HD et al., Nutr Metabol 2023, 20, 35). A comprehensive review of potential advantages in molecular characteristics, health benefits, and production techniques of conjugated linolenic acid vs conjugated linoleic acid has been published (Du M et al., J Agric Food Chem 2024, 72, 5503).
References on conjugated linoleic acid from 2000 may be found in the Mercola.com web site.
An estimation of the trans fatty acid content of foods and intake levels in France has been reported in 2007 (Laloux L et al., Eur J Lipid Sci Technol 2007, 109, 918).
The reformulation efforts in higher-income countries led to the World Health Organization target to eliminate industrially produced trans fatty acids globally. Thus, the World Health Organization set as a priority target the elimination of industrially produced trans fatty acids from the global food supply by the end of 2023 (Bruce JH, Nutr Bull 2023, 48, 427).
Conjugated linoleic acid (t9, t11-18:2, c9, t11-18:2 and t9, t11-18:2) can chemically be obtained from alkali-isomerized linoleate (Scholfield CR et al., JAOCS 1970, 47, 303). A mixture containing 72% c9,t11-18:2 and 26% c9,c11-18:2 was readily obtained through KOH-catalyzed dehydration of ricinoleic acid at 80°C with a 77% conversion efficiency (Yang L et al. Chem Phys Lipids 2002, 119, 23). The t10,c12-isomers may be prepared from the previous mixture by low-temperature crystallization in conjunction with urea treatment (Kim SJ et al., J Food Sci Nutr 2000, 5, 86). A preparation of trans,trans-isomers of linoleic acid may also be prepared by methylation with BF3/methanol in controlled conditions (Kim SJ et al., J Agric Food Chem 2003, 51, 3208). Position and configuration isomers of CLA, from 7,9- through 12,14-C18:2 were synthesized by direct sequential isomerizations of a mixture of rumenic acid and trans-10,cis-12 C18:2 (Destaillats F et al., Eur J Lipid Sci Technol 2003, 105, 3).
The products of these reactions usually differ in composition as they consist of a mixture of different isomers, which are currently sold as nutritional supplement. Tonalin® is one of the most sold brands of CLA.
The application of CLA-producing bacteria for the synthesis of CLA in food products is a challenging opportunity which is the aim of many investigations (Adamczak M et al., Eur J Lipid Sci Technol 2008, 110, 491).
Besides these C18 acids there is a shorter chain compound, sorbic acid.
Sorbic acid, or 2,4-hexadienoic acid, was first isolated in 1859 by AW von Hofmann from the unripe berries of the Rowan (Sorbus aucuparia). It was discovered in the late 1930s that they can be used as food preservative. Sorbic acid, and its sodium, potassium and calcium salts, are antimicrobial agents often used as preservatives in food and drinks to prevent the growth of mold, yeast and fungi (E200, E201, E202, E203). Sorbic acid may be obtained by thermal treatment or hydrolysis from parasorbic acid (a cyclic lactone of sorbic acid), also present in Sorbus aucuparia fruit. That compound is toxic (indigestion, nausea), but is easily transformed into sorbic acid by cooking.
|
Sorbic acid |
Sorbus aucuparia fruit |
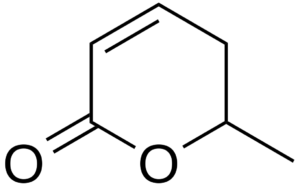
Parasorbic acid
Conjugated fatty acids are used in the production of alkyd resins to increase the drying time of normal vegetal oils.
Except rare examples in animals, conjugated polyene acids with more than two double bonds are mainly found in a few seed oils as mainly C18 trienes or tetraenes. The oils containing these fatty acids are very important raw materials in the manufacture of organic coatings and polymers, as the conjugated unsaturation facilitates good polymerization and imparts adhesive properties when properly treated. Conjugated trienes (c,t,t and t,t,t) are formed during the bleaching phase of commercial vegetable oils. Thus, they amount to 766 mg/kg in soybean oil, 654 in corn oil, 260 in safflower oil, 122 in sesame oil and 79 in rapeseed oil (Kinami T et al., JAOCS 2007, 84, 23). They are undetectable in olive oil. It has been reported that different forms of conjugated linolenic acid are present in cantaloupe and honeydew seeds at levels of about 2 mg/g seed kernel (Wang DH et al., J Agric Food Chem 2019, 67, 10306). These results suggest the potentially enhanced economic value of a specific class of bioactives that may be extracted from discarded food processing waste. The possible annual production of these products in USA has been estimated at about 37.5 tons.
The most common conjugated octadecatrienoic acids are :
– Calendic acid (8t10t12c-18:3) is found in Calendula officinalis (up to about 60% in Calendula oil) (Chisholm MJ et al., JAOCS 1966, 43, 391). An isomer (8t10t12t) was also detected in the same species. The same fatty oil, also contains small amounts of the isomer β-calendic acid, trans,trans,trans-8,10,12-octadecatrienoic acid.
 |
Calendula flowers are used for many centuries. Ointments or extracts are applied medicinally for reducing inflammation, wound healing, and as an antiseptic. Calendic acid is highly reactive and was proposed for several oleochemical applications : paints, coatings as a binder, as well as a reactive diluent. It can replace tung oil in commercial produced resins. The potential production capacity is about 3000 kg per ha with 21% oil in the seed. A follow-up program was started in Europe, the CARMINA project, focusing on possible industrial applications. |
– Catalpic acid (9t11t13c-18:3) is found in Catalpa ovata and C. bignonoides where it accounts for 50% of all the conjugated linolenic acid content (27.5% of the oil) (Ozgül-Yücel S, JAOCS 2005, 82, 893). Catalpa (Catalpa ovata) seed oil suppresses chemically-induced cell proliferation in rat colon (Suzuki R et al., Oncol Rep 2006, 16, 989). These results suggest that Catalpa oil could have chemopreventive activity in the early phase of colon carcinogenesis.
 |
Catalpa ovata is a pod-bearing tree native to China. Its height reaches between 20 and 30 feet (6 and 9 m). The inflorescences form 4–10-inch-long (100–250 mm) bunches of creamy white flowers with distinctly yellow tinging. They bloom in July and August. The leaves are very similar in shape to those of Paulownia tomentosa. |
– α–Eleostearic acid (9c11t13t) accounts for >65% of the fatty acids of tong (or tung) oil (china wood oil, Vernicia fordii, Euphorbiaceae), (the only source commercially available) and had an industrial importance. An isomer (9t11t13t) (β-eleostearic acid) was found in V. fordii, Momordica charantia (61%), Catalpa bignonoides (8.5%) and Punica granatum (21%) (Ozgül-Yücel S, JAOCS 2005, 82, 893). That isomer has been shown to induce apoptosis in T24 human bladder cancer cells through reactive oxygen species-mediated pathway (Sun Z. et al., Prostaglandins Other Lipid Mediat 2012, 99, 1).
 |
The tung tree is a species of Vernicia in the spurge family native to southern China, Burma, and northern Vietnam. It is a small to medium-sized deciduous tree growing to 20 m tall, with a spreading crown. The tung tree is valued for tung oil, which is derived from the seeds of the tree. Tung oil, also called China wood oil or nut oil, has traditionally been used in lamps in China. In modern times, it is used as an ingredient in paint, varnish, and caulk. It is also used as a wood finish for furniture and other wooden objects. The tung tree is poisonous in all of its parts, including the fruit and the seeds. |
α-Eleostearic acid is also found at a high level (about 65%) in oil from Parinarium excelsum seeds (Miralles J et al. Fatt Sci Technol 1994, 96, 64). A large survey of the distribution of this compound has revealed that it characterized the whole Chrysobalanaceae family, while in Rosaceae family only one species (Prunus mahaleb) was shown to contain this fatty acid (Ozgül-Yücel S, JAOCS 2005, 82, 893). Other sources include the seed oil of bitter gourd oil Momordica charantia (Cucurbitaceae) (50-65%). Nutrition experiments have shown that fatty acid could influence the levels of rat blood lipids (Dhar P et al., Lipids 1999, 34, 109). Anti-carcinogenic effects of this phytochemical have been particularly studied in in vitro and in vivo systems. Thus, α-eleostearic acid has been shown to exhibit a potential cytotoxicity and apoptosis induction effect on human breast cancer cells, with little effect on normal cells (Zhang T et al., J Nat Med 2012, 66, 77). The antitumor activity of α-eleostearic acid have been also focused on colon cancer (Yuan G-F et al., Food Func 2014, 5:1360).
– Jacaric acid (8c10t12c-18:3) is found in the seeds of Jacaranda mimosifolia (Bignoniaceae), a tree originating from Central and South America. The content of jacaric acid in the oil of these plants ranges from 30 to 36%. It displays strong cytotoxic effects on human adenocarcinoma cells in vitro (Shinohara N et al., Biochim Biophys Acta 2012, 1821, 980). It has also a strong preventive antitumor effect in vivo in nude mice into which these cells were transplanted and it has shown to have antiproliferative properties in vitro against prostate cancer.
 |
Flowers and fruits from Jacaranda mimosifolia. It is a sub-tropical tree native to south-central South America that has been widely planted elsewhere because of its attractive and long-lasting pale indigo flowers. They appear in spring and early summer, and last for up to two months. They are followed by woody seed pods, about 5 cm in diameter, which contain numerous flat, winged seeds. |
– Punicic acid (9c11t13c-18:3), known also as trichosanic acid, in the seed oil of Punica granatum (Punicaceae, Pomegranate), of Trichosanthes anguina (Cucurbitaceae, snake gourd), and of Momordica charantia (Punicaceae, bitter gourd). Pomegranate (Punica granatum) seed oil contains approximately 80% PuA and is currently the major natural source of this remarkable fatty acid.
Pomegranate seed oil is comprised up to 80% punicic acid. A new triacylglycerol containing two punicyl acyl groups and one acyl group (8c,11c,13t-18:3) was isolated from the seed of pomegranate (Punica granatum) (Yusuph M et al., Phytochemistry, 1997, 44, 1391).
Punicic acid has been shown to exhibit a myriad of beneficial bioactivities including anti-cancer, anti-diabetes, anti-obesity, antioxidant, and anti- inflammatory properties. It was shown to act physiologically as pro-oxidant or antioxidant according to the dietary level and to lower plasma cholesterol (Mukherjee C et al., J Oleo Sci 2002, 51, 513). It was also shown that this acid has an inhibitory effect in vitro on aggregation and arachidonic acid metabolism in human platelets (Takenaga M et al., Prostaglandins Leukot Essent Fatty Acids. 1988, 31, 65). As that fatty acid is very oxygen reactive, pomegranate oil may be use as binder or additives in coatings. Pomegranate seed oil was shown to suppress chemically induced colon carcinogenesis in rats (Kohno H et al., Cancer Sci 2004, 95, 481).
A comprehensive review of this fatty acid, covering topics ranging from its natural sources, biosynthesis, extraction and analysis, bioactivity, and industrial applications, to recent efforts and future perspectives on its production has been released (Holic R et al., Appl Microbiol Biotechnol 2018, 102, 3537). Health benefits of natural conjugated trienoic fatty acids had, in the recent years, encouraged the production of genetically modified plants with high level of punicic acid.
Application of punicic acid in therapy however poses some questions: it is slowly absorbed and it is rapidly metabolized forming other conjugated isomeric linolenic fatty acids such as catalpic and α- and β-eleostearic acids.
 |
The pomegranate originated from Iran to northern India, and has been cultivated since ancient times throughout the Mediterranean region. The fruit is typically in season in the Northern Hemisphere from September to February. As intact arils or juice, pomegranates are used in baking, cooking, juice blends, meal garnishes, smoothies, and alcoholic beverages, such as cocktails and wine. Botanically, the edible fruit is a berry with seeds and pulp produced from the ovary of a single flower. In India’s traditional medicine, the pomegranate is frequently described as an ingredient in remedies.
|
Another conjugated triene fatty acid, rumelenic acid (cis-9,trans-11,cis-15 18:3), has been described as a minor component in ruminant fats (Destaillats F et al., J Dairy Sci. 2005, 88, 3231). That conjugated fatty acid is an intermediate of the “biohydrogenation” process of a-linolenic (cis-9,cis-12,cis-15 18:3) acid in the rumen. An isomer (cis-9,trans-13,cis-15 18:3) was also detected at low concentration in milk fat.
The methyl ester of 2t,4c,6c-10:3 was identified as a sex-specific compound from the stink bug Thyanta pallidovirens (Millar JG, Phytochemistry, 1997, 38, 7971).
One conjugated tetraene from Parinarium sp (Rosacae from west Africa) and Impatiens balsamina, parinaric acid (α-parinaric: 9c11t13t15c and b-parinaric: an all-trans species) is used in biophysical studies to measure the ordering (rigidity) of lipidic membranes. It was discovered in 1933 (Tsujimoto M et al., J Soc Chem Ind Japan 1933, 36, 110B) and its exact structure reported in 1935 by Farmer EH (J Chem Soc 1935, 759). The presence of parinaric acid was confirmed in the seed oil of Sebastiana brasiliensis (Euphorbiaceae) was confirmed by a combination of physico-chemical methods (Spitzer V et al., JAOCS 1996, 73, 569).
 |
Impatiens balsamina, commonly known as balsam, garden balsam, is a species of plant native to India and Myanmar. It is an annual plant growing to 20–75 cm tall, with a thick, but soft stem. The leaves are spirally-arranged, with a deeply toothed margin. The flowers are pink, red, mauve, lilac, or white; they are pollinated mainly by bees and other insects. The ripe seed capsules undergo a well known explosive dehiscence. It is widely cultivated as an ornamental plant. Different parts of the plant are used in various countries as traditional remedies. |
Two new eicosapentaenoic acids, 5c,7t,9t,14c,17c-20:5 and 5t,7t,9t,14c,17c-20:5 were detected in the free fatty acid fraction extracted from the temperate red marine alga, Ptilota filicina (Ceramiales, Rhodophyta) colected in the Oregon coastal waters (Lopez A et al., Lipids 1987, 22, 190).
More recently, novel polyene fatty acids with four conjugated double bonds were found in a marine green microalga, Anadyomene stellata (Mikhailova MV et al., Lipids 1995, 30, 583). Five different fatty acids with different chain lengths and varying unsaturation were described: 16:5, 18:4, 20:5, 20:6, and unexpectedly 22:7. All these species have in common 4 conjugated all-cis double bonds as in 18:4 with their position in 6,8,10, and 12, the novel conjugated docosaheptadecanoic acid having its double bonds in 4, 7, 9, 11, 13, 16, and 19, it was named stellaheptaenoic acid.
The expression of cDNAs for variant forms of the delta12-oleic acid desaturase in transgenic soybean embryos resulted in the production of conjugated polyenes (Cahoon EB et al., PNAS 1999, 96, 12935).
A review has summarized and updated the evidence regarding the metabolism and bioactivities of conjugated linolenic acid, and discussed the recent studies on the effects of anti-carcinogenic, lipid metabolism regulation, anti-inflammatory, anti-obese and
antioxidant activities of the variousisomers (Yuan GF et al., Food Funct 2014, 5, 1360).
![]()
Among the unsaturated polymethylene-interrupted fatty acids (also known as non-methylene-interrupted fatty acids) those with a cis-5 ethylenic bond (D5-olefinic acids) are found in various sources in the plant kingdom but may now be considered characteristic components of Gymnosperm seed oils (Wolff RL et al., J Lipid Res 1996, 73, 765).
In the genus Pinus as well as the whole family Pinaceae, the same 5-unsaturated polymethylene-interrupted fatty acids are found (review : Wolff RL et al., Lipids 2000, 35, 1). The three most frequent fatty acids with that structure are taxoleic acid (all-cis-5,9-18:2), pinolenic acid (all-cis-5,9,12-18:3) which is found in seeds of conifers as Taxaceae and present in most taxa of Pinus (Pinaceae) (Lahlou A et al., Phytochemistry 2023, 206, 113517), Teucrium and also in tall oil (by-product in pine wood processing), and sciadonic acid (all-cis-5,11,14-20:3).
These fatty acids are present in seed oil at levels from about 1% up to 25%.
Pinolenic acid reached a maximum of 28.3% of total fatty acids in Pinus mugo (Lahlou A et al., Phytochemistry 2023, 206, 113517). Pinolenic acid was shown to be also present in Korean pine nut oil (Pinus koraiensis) from where it was precisely studied after several purification steps (Lee JW et al., Lipids 2004, 39, 383). That study suggested that pinolenic acid may have LDL-lowering properties by enhancing hepatic LDL uptake. It was shown that these fatty acids are able to reduce plasma triglycerides and VLDL in rats (Asset G et al., Lipids 1999, 34, 39). The health benefits of pinolenic acid have attracted extensive attention and been widely researched. The positive effects have been reported in various fields, including anti-inflammatory, antioxidant, metabolic regulation, prevention of atherosclerosis, and potential anti-cancer properties. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid has been published (Baker EJ et al., Prog Lipid Res 2021, 82, 101097).
Sciadonic acid was shown to be present in seeds and leaves of all Coniferophytes examined (including conifers and ginkgoids) (Wolff RL, JAOCS 1999, 76, 1515). In contrast, angiosperms have lost the ability to introduce a supplementary desaturation at C-5 in unsaturated fatty acids. A diagnostic index based on the relative levels of the main fatty acids including distinctive D5-olefinic acids was used to identify the botanical origin of pine nuts in food products (Destaillats F et al., J Agric Food Chem 2010, 58, 2082).
Naturally occurring 5,9-fatty acids include also the shorter-chain analog 5,9-16:2 and 5,9-18:2 which were reported from several cellular slime molds. A new triene fatty acid desaturated at C5, all cis-5,9,12-17:3, was isolated from the cellular slime mold Polysphondylium pallidum (Saito T et al., Lipids, 1996, 31, 445). A taxoleic elongation product, dihomotaxoleic acid (7,11-20:2), has been characterized in Taxus seed lipids containing also high amounts of taxoleic acid (Destaillats F et al., Lipids 2001, 36, 319).
Similar species with 4 double bonds are also described.
Another curious fatty acid was described in the pulp lipids of mango (Mangifera indica), a butylene-interrupted dienoic acid, cis-9, cis-15 octadecadienoic acid (9,15-18:2). It amounts to about 5.4% of total acyl groups in the pulp lipids (Shibahara A et al., Biochim Biophys Acta 1993, 1170, 245).
A new octadecatrienoic acid, all-cis-3,9,12-18:3, was found a component of an Asteraceae (Chrysanthemum zawadskii) seed oil (6.9%), in addition to crepenynic acid (8.6%) and other common fatty acids (Tsevegsuren N et al., Lipids 2003, 38, 573). The seed oil of Aster scaber has been showned to contain considerable amounts of Δ3t-16:1 (11.4%), Δ3t, 9c-18:2 (4.6%), and Δ3t, 9 c, 12c-18:3 (11.3%) (Choi YS et al., J Oleo Sci 2015, 64, 1321).
(3E,7Z)-Tetradecadienyl acetate was determined to be the major sex pheromone component of the potato pest Symmetrischema tangolias (Awalekar R et al., Chem Nat Compd 2021, 57, 1000–1004).
In lower animals, a group of C18 up to C31 acids are present in many sponge species and are characterized by the presence of 5c9c unsaturation, also often accompanied by other functionality. These compounds are present in freshwater sponges and in the marine sponges from the class Demospongia and are known as demospongic acids, mainly 5,9,17-26:3, 5,9,21-28:3, and 5,9,23-30:3 (Review in : Dembitsky VM et al., Chem Phys Lipids 2003, 123, 117). As an example, 12 of these fatty acids and a novel one (5,9,22-29:3) have been described in the sponge Verongia aerophoba from the Canary islands (Nechev J et al., Eur J Lipid Sci Technol 2002, 104, 800). It was suggested that the presence of demospongic acids in lipid extracts from gorgonian coral species reflects the presence of a symbiotic sponge (Imbs AB et al., Lipids 2009, 44, 325).
Such acids have also been observed in various marine sources with a large range of chain-lengths (C16 to C32) and from some terrestrial plants with short acyl chains (C18 to C19). Finally, the Δ5,9 fatty acids appear to be a particular type of non-methylene-interrupted fatty acids. An article reviewed the occurrence of these particular fatty acids in marine and terrestrial organisms (Kornprobst JM et al., Mar Drugs 2010, 8, 2569).
The use of an antiplasmodial bioassay revealed that fatty acids with 23 to 26 carbon atoms and double bonds in the position Δ5,9 displayed considerable antiprotozoal activity (IC50 : 12-16 mg/ml) (review in Carballeira NM, Prog Lipid Res 2008, 47, 50). The mechanism of action of these compounds is intensely studied (Tasdemir D et al., Bioorg Med Chem 2007, 15, 6834). Thus, these sponge fatty acids may be the source of very potent antimalarial drugs. These fatty acids are also potent inhibitor of the enzyme topoisomerase I, property which must lead to the development of effective anti-cancer drugs.
The predominant presence of demospongic acids in phospholipids (and in particular the amino-phospholipids) (Lawson MP et al., Lipids 1988, 23, 741) challenges the hypothesis of an unique structure for sponge cell membranes.
It has been demonstrated that sponges are not the only source of these 5,9 dienoic acids, since they were found to be present in other marine organisms, such as zoanthids and anemones. It is likely that these fatty acids arise from a symbiotic relationship of bacteria with the host cells of marine invertebrates. Two 5,9 trienoic acids have been identified for the first time in triacylglycerols from mollusk gonads, 5,9,15-22:3 and 5,9,15-24:3 (Kawashima H, Lipids 2005, 40, 627).
While all these unsaturated fatty acids were isolated from phospholipids, a polyethylenic fatty methyl ester (5,9,23-30:3) was isolated from a Mediterranean sponge (Chondrilla nucula, Demospongiae) (Meyer M et al., Lipids 2002, 37, 1109).
Based on the distribution of 5,9 fatty acids it may conclude that the biosynthetic pathways of invertebrates, sponges, myxomycetes, and some plants have a common enzymatic system to synthesize 5,9 ethylene-interrupted dienoic acids. It can thus be observed that the former notion of demospongic acid should no longer be used mainly because bis-methylene interrupted 5,9-diunsaturated fatty acids are distributed among several phyla of marine organisms and several classes of terrestrial plants (Kornprobst JM et al., Mar Drugs 2010, 8, 2569). As these authors suggest, the group of “demospongic acids” can be considered as a particular series of non-methylene-interrupted fatty acids produced by different combinations of elongases and D5 and D9 desaturases on the most common fatty acids in nature such as palmitic and palmitoleic acids.
Four types of non-methylene-interrupted polyunsaturated fatty acids were found at concentrations of 2-13% of polar and neutral lipids in Ophiuroidea (Brittle star) : 7t,13t-20:2, 7t,13t,17c-20:3, 9c,15c, 19t-22:3, and 4t,9c,15c,19t-22:4 (Sato D et al. J Oleo Sci 2002, 51, 563). Several others have been identified in limpet gonads, among them 5,11-19:2, 7,16-21:2, 9,15-24:2, 5,11,14,17-20:4 and 7,13,16,19-22:4 were reported for the first time (Kawashima H, Lipids 2005, 40, 627). A great number of dienoic non-methylene-interrupted fatty acids have been described in marine molluscs and echinoderms, some amounting up to 20% of membrane lipids (Barnathan G, Biochimie 2009, 91, 671).
Novel n-4 non-methylene fatty acids have been isolated from a clam, Calyptogena phaseoliformis, living near deep hydrothermal vents (Saito H, J Chromatogr A 2007, 1163, 247). With some others (7,15-20:2, 7,16-21:2), these fatty acids are 5,13,16-20:3, 4,7,15-20:3, 1,4,7,15-20:4 and 4,7,16-21:3. They were isolated also from cold-sep mussels, hosting methane-oxidizing bacteria (Saito H, J Chromatogr A 2008, 1200, 242). The origin of these unique fatty acids is likely the symbiotic chemosynthetic bacteria belonging to the communities using geothermal energy and nutrients originating from he vents.
Unusual non-methylene interrupted fatty acids were also isolated from a marine mollusk, Crepidula fornica : 7,15-22:2 and 9,13-18:2 (Dagorn F et al., 2009).
Four novel non-methylene fatty acids with a terminal double bond [7,18-nonadecadienoic (19:2Δ7,18), 11,18-nonadecadienoic (19:2Δ11,18), 7,20-heneicosadien- oic (21:2Δ7,20), and 11,20-heneicosadienoic (21:2Δ11,20) acids] have been detected in the ovaries of a marine mollusk, the Limpet Cellana toreuma (Kawashima H et al., Lipids 2017, 52, 375). From these findings, possible biosynthetic pathways for these novel fatty aids have been discussed.
A novel polymethylene-interrupted fatty acid, 22:3(7Z,13Z,16Z), derived from sciadonic acid has been identified in a marine worm (Urechis unicinctus) consumed primarily in parts of China, Korea, and Japan (Yang H et al., Food Chem 2025, 463, 141287). Some other polyethylene-interrupted PUFA, i.e., 20:2(5Z,11Z), 20:2(5Z,13Z), 22:2(7Z,13Z), 22:2(7Z,15Z), 20:3(5Z,11Z,14Z) have also been detected.
The saponification of a highly unsaturated lipid fraction of Mycobacterium phlei liberates mainly an unusual 36 carbon fatty acid, hexatriaconta-4,8,12,16,20-pentaenoic acid and several others with the common formula :
CH3-(CH2)m-(CH=CH-CH2-CH2)n-COOH
when m=14, n=4, 5 or 6
when m=12, n=5 or 6
The name phleic acid was given to these acids because they were all isolated from M. phlei. It was found that they always occur as esters of trehalose. Their original biosynthesis has been reported (Asselineau CP et al., Eur J Biochem 1976, 63, 509).
![]()
Allenic acids contain the -CH=C=CH- group. They occur only rarely in natural lipids.
The first allenic acid (monoacid) to be identified in the seed oil of Leonotis napetaefolia (Labiateae) was laballenic acid (5,6-octadecadienoic acid) (Bagby MO et al., J Org Chem 1965, 30, 4227). Two C18 allenic acids were described (Hagemann JM et al., Lipids 1967, 2, 371) in seed oils of several members of Labiateae (mint family) : 5,6-18:2 (laballenic acid) and 5,6,16-18:3 (lamenallenic acid). The seed oil of another Labiateae Leucas cephalotes with 28% of laballenic acid is the richest source of this compound (Sinha Set al., Chem Ind 1978, 67).
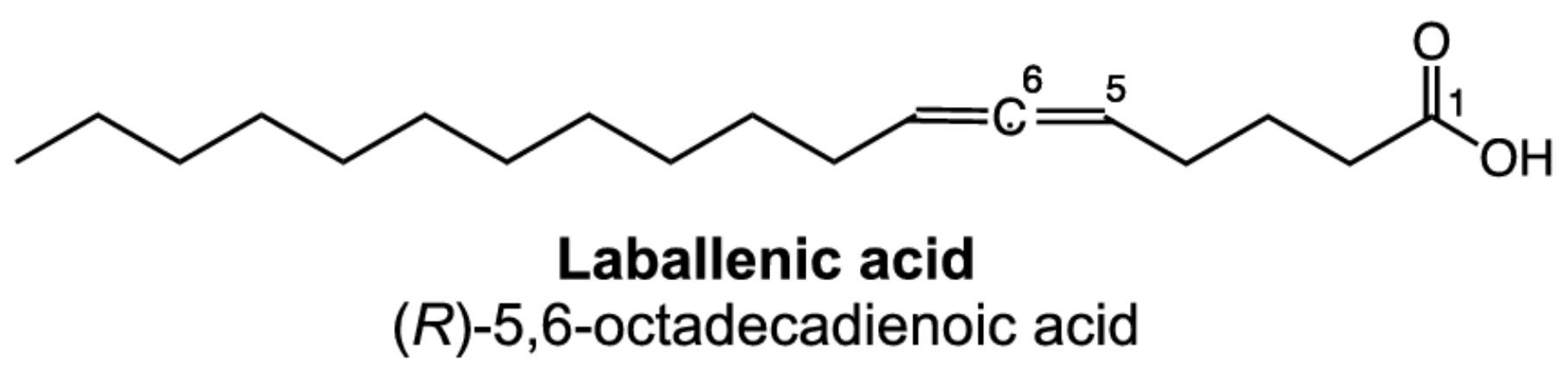
Lamenallenic acid (16% of total fatty acids) has been detected in Lamium purpureum.
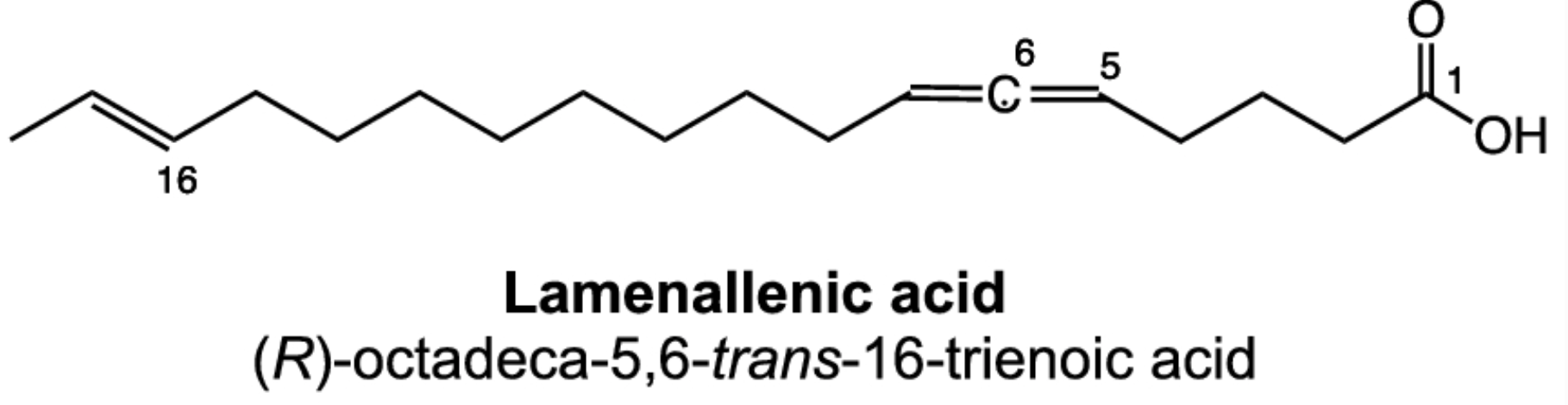
A triene allenic fatty acid with 14 carbon atoms (2,4,5-14:3) was shown to occur as a sex pheromone in the male dried bean beetle.
A hydroxy allenic acid (8-hydroxy-5,6-octadienoic acid) was described in an estolide found in some plants.
A review of known allenic acids may be consulted for further details (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
Cumulenic fatty acids contain the –HC=C=C=CH– group. The first cumulenic acids, such as two g-lactones of 4-hydroxy-2,4,6,7,8-decapentaenoic and 4-hydroxy-2,4,5,6,8-decapentaenoic acids were isolated from several Asteraceae (Bohlmann F et al., Chem Ber 1971, 104, 1329).
The 2,6,7,8-decatetraen-4-ynoic acid, 9-(methylthio)-, methyl ester (269) was also obtained from an Asteraceae Anthemius austriaca.
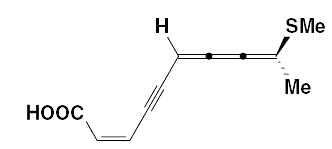
Other cumulenic fatty acids (among them 2,3,4-decatrienoic acid) were isolated from Matricaria inodora.
A review of cumulenic lipids may be consulted for further details (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire