
Sesquiterpenoids are defined as the group of 15 carbon compounds derived by the assembly of 3 isoprenoid units and they are found mainly in higher plants but also in invertebrates. Sesquiterpenes, with monoterpenes, are an important constituent of essential oils in plants. They are the most diverse group of terpenoids and may have acyclic (linear), monocyclic, bicyclic, or tricyclic frameworks, showing many unique arrangements. Sesquiterpenes can occur as hydrocarbons or contain oxygen functionality, including carboxylic acids, lactones, alcohols, aldehydes, ketones, and epoxides. Chain extension with augmentation in a number of cyclizations as well as biochemical modifications (rearrangement or oxidation) allows a good variety of structures from the sesquiterpenes.
In plants, they function as pheromones and juvenile hormones. Several are known to have immunomodulatory activity. Studies have revealed a new mechanism for plant–tree interactions in a boreal ecosystem: Rhododendron tomentosum-emitted volatile sesquiterpenes are passively adsorbed to Betula spp. foliage. When re-released, these volatiles were repellent to birch herbivores, which suggest the presence of associational resistance. This could be ecologically important, affecting plant fitness and even species distribution (Himanen SJ et al., New Phytologist 2010, 186, 722).
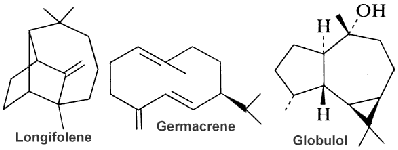
Some of natural sesquiterpenoids are shown below.
The acyclic representative are also called farnesans, term derived from the basic structure, farnesol. Farnesol and nerolidol are very common and are isolated from essential oils of various sources.
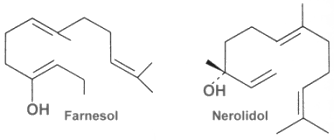
Farnesol is widely distributed in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, and balsam. It is used in perfumery to emphasize the odors of perfumes. Moreover, it is a natural pesticide for mites and is also a pheromone for several insects and mammals, including elephants (teritorial marking, individual recognition, mate attraction). Farnesyl acetate is found in the web of some spiders (Pholcidae) to attract females (Schulz S, J Chem Ecol 2013, 39, 1). Farnesol was also show to be the “quorum-sensing molecule” identified in fungi (Hornby JM et al., Appl Environ Microbiol 2001, 67, 2982). The presence of farnesol prevents the yeast-to-mycelium conversion, resulting in actively budding yeasts without influencing cellular growth rates. This study is the first to identify an extracellular molecule mediating an eukaryotic quorum-sensing system.
Farnesol is active against a variety of Candida albicans at concentrations between 1 and 50 mM (Hornby JM et al., Antimicrob Agents Chemother 2003, 47,2366).
Farnesol is frequently esterified with one fatty acid having 8 to 12 carbon atoms.
Farnesene, an analogue of farnesol, is a collective name that refers to four stereoisomers of alpha-farnesene and two stereoisomers of beta-farnesene (Wikipedia). It is known to act as an alarm pheromone in aphids. Released during predator attack, it causes aphids to stop feeding, disperse, and give birth to winged (rather than wingless) forms, which leave their host plants. Farnesene is produced by de novo biosynthesis by cotton plants when damaged by insect herbivories (Paré PW et al., Plant Physiol 1997, 114, 1161). These compounds likely mediate the interaction between herbivores and their natural enemies, attracted by terpenes.
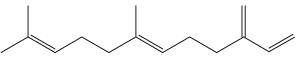
Farnesene
Microbially produced farnesene has been used as a precursor of polymers for the tire industry and the fully hydrogenated compound, farnesane, has been evaluated as a possible diesel additive. Amyris has produced the fuel Biofene® derived by chemical hydrogenation of farnesene.
Nerolidol is present in neroli, ginger, jasmine, lavender, tea tree and lemon grass. The aroma of nerolidol is woody and reminiscent of fresh bark. It is used as a flavoring agent and in perfumery. It was also shown to be produced by the leaves of a large number of plant species in response to herbivory insects and then to be transformed into a C11-homoterpene (4,8-dimethyl-1,3,7-nonatriene) which attracts predatory insects (Dicke M et al., J Chem Ecol 1990, 16: 3091–3118).
4,8-Dimethyl-1,3,7-nonatriene
Sesquiterpenes, with acetoxy and aldehyde functionalities have been shown to be potent feeding deterrents and in some cases cytotoxic towards predatory species of herbivorous fish (Sun HH et al., Tetrahedron Lett 1979, 8, 685). It has been suggested that caulerpenyne, found in Caulerpa trifaria, is a key player in the cytotoxicity of Caulerpa algae towards herbivores. This sesquiterpene appears to contribute to the invasiveness of the genus Caulerpa likely by means of its inhibition of the key organic anion transporters.
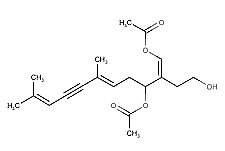 Caulerpenyne
Caulerpenyne
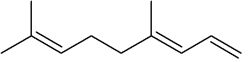
Among the acyclic species, two compounds are well known for their importance in invertebrate endocrinology. One, methyl farnesoate is now considered as the crustacean juvenile hormone (Homola E et al., Comp Biochem Physiol 1997, 117B, 347) and is synthesized by the mandibular organs and is present in the hemolymph. Its structure is nearly identical to the other one, the insect juvenile hormone III which regulates metamorphosis and reproduction. Both are synthesized from farnesoic acid in the corpora allata.
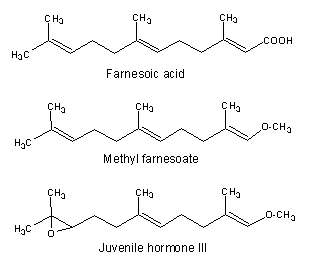
Farnesoic acid has been determined to be the autoregulatory substance involved in the regulation of the morphological transition of the yeast Candida albicans between a budding form and a multicellular invasive filamentous form (Oh KB et al., Proc Natl Acad Sci USA 2001, 98, 4664). This transformation has been postulated to contribute to the virulence of this organism. These findings might have medicinal value in the development of anti-fungal therapies.
4,8,12-Trimethyltrideca-1,3,7,11-tetraene (or TMTT) is a volatile compound produced from a number of plant species, including corn, lima bean, tomato, and many other plant species in response to herbivore damage. It may play a role in attracting predators to combat the herbivorous attackers. Thus, the chemical and molecular basis of the key herbivore-induced volatile TMTT in mediating tomato–whitefly (Bemisia tabaci)–parasitoid interactions and its potential for augmenting biological control were elucidated (Bai Y et al., J Agric Food Chem, February 12, 2026 on line).
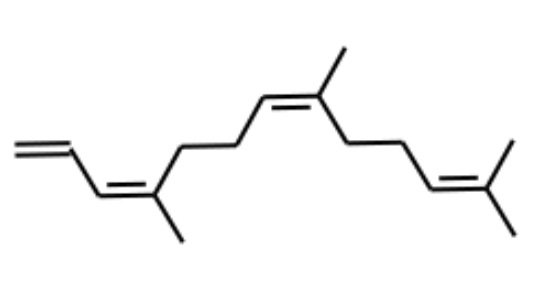
4,8,12-Trimethyltrideca-1,3,7,11-tetraene
Abscisic acid plays a key role in plants in the regulation of stomatal closure by regulating ion channel activities and water exchanges across the plasma membrane of guard cells. It was originally believed to be involved in leaf abscission but this is now known to be the case only in a small number of plants.
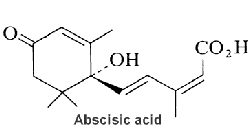
Cyclic ADP-ribose (cADPR) has been shown to mediate signaling of abscisic acid in the drought-stress response leading to activation of gene transcription and to stomatal closure (Leckie C P et al., Proc Natl Acad Sci U S A 1998, 95, 15837). It was shown that diacylglycerol pyrophosphate plays a role as phospholipid second messenger in abscisic signaling (Zalejski C et al., Plant J 2005, 42,145). A review of the signaling network may be found in a paper by Giraudat J (Curr Opin Cell Biol 1995, 7, 232)and in the “Plant hormones” textbook (Litwack G Ed, Elsevier, 2005). Abscisic acid is an end product of neoxanthin or violaxanthine peroxidation and reduction giving an apocarotenal (apocarotenoid) with a short side chain (5 carbons), followed by a final oxidation into an acid form (Seo M et al., Trends Plant Sci 2002, 7, 41).
Abscisic acid has also a variety of roles in plant development, bud and seed dormancy, germination, cell division and movement. It induces also storage protein synthesis in seeds and may be involved in defense against insect attack. Unlike the structurally related sesquiterpenes, which are formed from the mevalonic acid-derived precursor farnesyl diphosphate, abscisic acid is biosynthesized via carotenoids (zeaxanthin, neoxanthin, violaxanthin) in roots and mature leaves. Its direct precursor is xanthoxin which is a natural inhibitor of plant growth. Abscisic acid concentration was found in the stigma exudate of Nicotiana tabacum and it strongly stimulated the germination of tobacco pollen, a lower concentration had a weaker effect, increasing the concentration did not increase the effect (Breygina M et al., Biomolecules 2023, 13(9), 1313).
Abscisic acid is ubiquitous in lower and higher plants, it is present also in algae. Only the C2-cis, C4-trans isomer is biologically active. A mechanism of abscisic signaling in connection with cyclic ADP-ribose and calcium movement has been demonstrated to mediate temperature signaling in sponges (Zocchi E et al., Proc Natl Acad Sci USA 2001, 98, 14859) as well as tissue regeneration in Cnidaria (Puce S et al., J Biol Chem 2004, 279, 39783). Seed germination is inhibited by abscisic acid in antagonism with gibberellin. Although not well understood, growing evidence indicates an antagonistic interaction between the ethylene and abscisic acid pathways in regulating ethylene and abscisic acid production (Li Z et al., Plant Signal Behav 2011, 6, 1647).
Abscisic acid is not restricted to the plant kingdom and primitive invertebrates since it has been shown to be present in the central nervous system of pigs and rats (Le Page-Degivry MT et al., Proc Natl Acad Sci USA 1986, 83, 1155). Evidence was provided that it is also involved in the stimulation of human granulocytes with cyclic ADP-ribose as second messenger (Bruzzone S et al., Proc Natl Acad Sci USA 2007, 104, 5759). This lipid may be considered as a new pro-inflammatory cytokine in humans. Human granulocytes and smooth muscle cells are also functionally activated by abscissic acid (Magnone M et al., J Biol Chem 2009, 284, 17808). That messenger can be considered as a new signal molecule involved in the development of atherosclerosis.
Abscisic acid was shown to be a endogenous stimulator of insulin release from human pancreatic b cells with cyclic ADP-ribose as second messenger (Bruzzone S et al., J Biol Chem 2008, 283, 32188). This observation suggests that this lipid phytohormone may be involved in the physiology of insulin release, mainly in its dysregulation under conditions of inflammation. Together with jasmonates, abscisic acid was shown to control excitability and closure of the insect trap of Dionaea muscipula (Venus flytrap) (Escalante-Perez et al., PNAS 2011, 108, 15492).
Several studies have shown that several male insects, such as Tibraca limbativentris (Hemiptera: Pentatomidae), use zingiberenols as sex pheromone components (Blassioli-Moraes MC et al., J Chem Ecol 2020, 46, 1). As these insects are threatening rice production, several studies are devoted to the study of these compounds, being potentially used for protection of rice plants in cultivated areas. Zingiberenol is a constituent of ginger (Zingiber officinale).
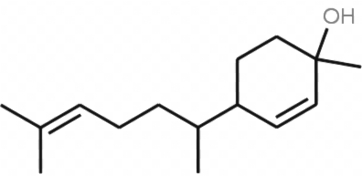
Zingiberenol
Cadalene has the cadinane skeleton and is present in essential oils and in many plants. It is used as a biomarker in paleobotanic studies. In connection with retene (1-methyl-7-isopropyl phenanthrene), it enables the estimation in sediments of the importance of Pinaceae in ancient forests.
Some important sesquiterpenes
Cadalene
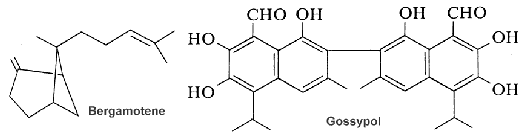
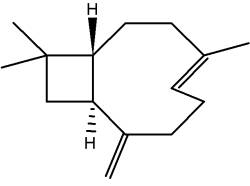
The bergamotane family (see bergamotene in the figure) represents an uncommon class of natural sesquiterpenes that includes bi-, tri-, or tetracyclic derivatives. Bergamotane sesquiterpenoids having a bridged 6/4 bicyclic skeleton involved in an isopentyl unit are biosynthesized by fungi and plants (Fraga BM, Nat Prod Rep 2013, 30, 1226). Bergamotane sesquiterpenoids have been reported from various marine sources such as sponges, sea mud, deep-sea hydrothermal sulfide deposits, and sea sediments. These metabolites could gain the interest of chemists and biologists because of their unusual structural features and diversified activities, such as phytotoxicity, plant growth regulation, antimicrobial, anti-HIV, cytotoxic, pancreatic lipase inhibition, immunosuppressive, antidiabetic, and anti-inflammatory properties (Khayat MT et al., Mar Drugs 2022, 20, 771).
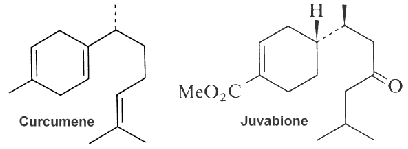
The function of curcumene as insecticide, repellent, and insect feeding deterrent has been previously reported. A parent compound, bisabolol, isolated from essential oils of a variety of plants, showed at low concentration a differentiating effect in endothelial cells but an apoptotic effect at higher concentration (Magnelli L et al., J Nat Prod 2010, 73, 523).
Germacrene is typically produced in a number of plant species for its antimicrobial and insecticidal properties, though it also plays a role as insect pheromones. It has shown antiviral properties in an animal model of influenza infection (Liao Q et al., Antiviral Res 2013, 100, 578).
Germacrene has five isomers (A to E). It is present in tobacco and protects it against parasite insects.
Gossypol, a sesquiterpene dimer found in cotton that is formed from two cadinane units. All the cotton plant contains gossypol. That terpene occurs as a mixture of two enantiomers but each has different biological activities. For nonruminant animals (rodents, chickens, humans), (–)-gossypol is significantly more toxic than the (+) enantiomer. It has anti-cancer properties and inhibits male fertility in humans. In contrast, cotton plants containing high levels of (+)-gossypol are resistant to insect damage. These terpenes must be removed from the plant parts and oil before use as animal foods.
Juvabione, historically known as the ”New York Times paper factor”, has been found in the wood of true firs of the genus Abies. As some other sesquiterpenes, juvabione is an insect juvenile hormone analogues because it has the ability to mimic juvenile activity in order to stifle insect reproduction and growth (Bohlmann J et al., Proc Natl Acad Sci USA 1998, 95, 6756). This compound plays an important role in conifers as the second line of defense against insect induced trauma and fungal pathogens.
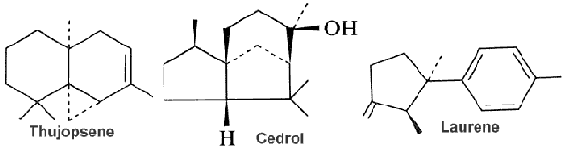
Cedrol is found in the essential oil of conifers (cedar oil), especially in the genera Cupressus (cypress) and Juniperus (juniper). Its main uses are in the chemistry of aroma compounds. It makes up 16-19% of cedarwood oil and is used in traditional medicine and cosmetics. Methyl cedryl ether, also recognized as cedramber, is synthesized by methylation of cedrol and is widely used in cosmetic industries as a fragrance compound. It was also shown that it could have preventative effects on obesity and related metabolic syndromes (Li M et al., Nutrients 2023, 15, 788).
Bicyclic sesquiterpenes with a driman unit are widespread in plants, liveworts, fungi and certain marine organisms (sponges) (Jansen BJ et al., Nat Prod Rep 2004, 21, 449). Some drimanes have been identified in petroleum and are probably derived from a microbial source (Noble RA et al., Org Geochem 1987, 11, 151).
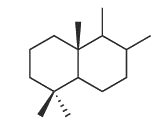
Driman squeleton
They have generally potent antibacterial and antifungal activities, and they are toxic to several invertebrates and also in fish. In addition, they deter feeding by insects on plants and by fish on sponges.
The acidic fraction of Costus root oil is composed of several acids, one of which, costic acid, has been obtained in the pure crystalline form (Bawdekar AS et al., Tetrahedron 1965, 21, 1521). It is the acid corresponding to costol, the selinenic allylic alcohol previously isolated. Treatment with costic acid was shown to reduce the inflammatory parameters with a decrease in the levels of inflammatory cytokines and chemokines (Ferreira BA et al., Fitoterapia 2024, 175, 105939). This compound caused also a reduction in angiogenic parameters, such as a decrease in the number of blood vessels, the hemoglobin content, and the levels of VEGF and FGF cytokines.
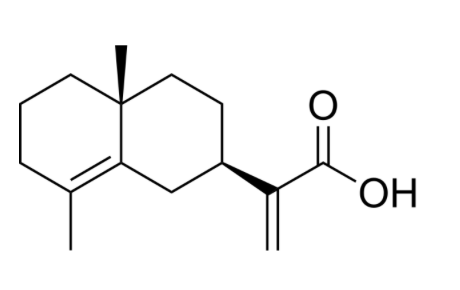 Costic acid
Costic acid
Geosmin is an irregular sesquiterpenoid, produced from the precursor farnesyl pyrophosphate. Its name is derived from the Ancient Greek words geō-, meaning “earth”, and osmḗ, meaning “smell” as it accounts for the majority of odor outbreaks in drinking water worldwide and for the muddy smell in many freshwater fish such as carp, catfish, and tilapia. Geosmin has a distinct earthy or musty odor, which most people can easily smell. Its detection threshold in humans is very low, about 0.01 micrograms per liter in water. It is also responsible for the earthy taste of beetroots and a contributor to the strong scent (petrichor) that occurs in the air when rain falls.
 Geosmin
Geosmin
Geosmin is produced by various blue-green algae (cyanobacteria: Anabaena, Phormidium, and Planktothrix) and filamentous bacteria (Actinomyces: Streptomyces).
Capsidiol is a sesquiterpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. It is considered as a phytoalexin.
Capsidiol
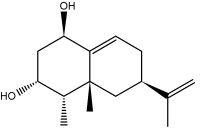
Eremophilanes are biologically active bicyclic sesquiterpenoids extracted from the essential oil of plants of the genus Eremophila (family Myoporaceae), notably Eremophila mitchellii (Beattie K et al., Phytochemisry 2011, 72, 400).
Eremophilane is a bicyclic sesquiterpene isolated from wood, leaf, branch and root oils of Eremophila mitchellii. known also as false sandalwood, is a flowering plant in the Myoporacaea family and is endemic to Australia. Aboriginal people used that plant to treat rheumatism and the smoke from burning the leaves for various medicinal purposes.
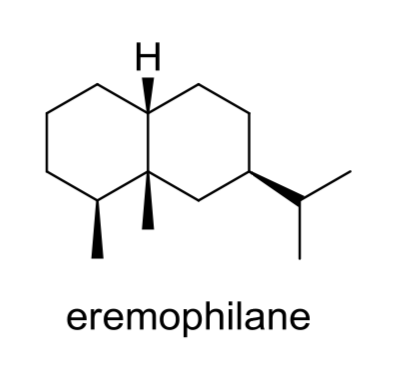
Several molecules having the eremophilane structure have been described in other plants of several genera (such as Ligularia, Senecio, Cacalia) of Asteraceae and fungi and due to their insecticidal and termiticidal properties there is a resurgence of interest into the commercial production of the wood oil of E. mitchellii. These compounds possess a number of biological activities such as anti-tumor, anti-bacterial and anti-inflammatory, and thus they have received increasing interest in the recent years (Hou C et al., Mini-Rev Med Chem 2014, 14, 664 – 677). An anti-neuroinflammatory eremophilane sesquiterpenoids has been isolated from marine-derived fungus Phoma sp. DXH009 (Yang G et al., Mar Drugs 2025, 23, 94). The sesquiterpene lactone eremophila-1(10),11(13)-dien-12,8β-olide, isolated from leaves of the Calomeria amaranthoides plant (Asteraceae) which is endemic to New South Wales and Victoria, Australia. It has cytotoxic activity on human cancer cell lines, including ovarian cancer-derived cells (van Haaften C et al., J Nat Prod 2025, 88, 1766).
11,12-Dihydrolactaroviolin was initially isolated from the edible mushroom Lactarius deterrimus (Russulaceae family) (Koul SK et al., Phytochem 1985, 24, 181) and was subsequently found to be widely present in Lactarius species. It was later recognized as an antibacterial compound which selectively inhibited Mycobacterium tuberculosis, including 80 drug-resistant strains, with a minimum inhibitory concentration of 7.8 μg/mL. 500 μg/mL of dihydro-LAC showed no significant cytotoxicity to normal cells (Zhon P et al., Food Chem 5 November 2025, 146964). This terpene undergoes rapid enzymatic alterations upon tissue injury, making it difficult to quantitate.
 11,12-Dihydrolactaroviolin
11,12-Dihydrolactaroviolin
Avarol, and its quinone derivative avarone, are biologically active sesquiterpenoids which exhibited antimicrobial and antifungal activities and also active against AIDS virus. They were isolated from a Mediterranean (Dysidia avara) and an Australian sponge (Dysidia spp). They have also potent antileukemic activity both in vitro and in vivo in mice.
Avarol
The presence of bibenzyls (compounds with two aromatic rings), a unique group of phenolic molecules present in oil bodies liverworts, are abundant in bryophytes, with a structural diversity. Mosses are generally devoid of these molecules, The first bis bibenzyls were discovered in 1982 (Asakawa Y et al., Phytochemistry 1982, 21, 2481). Since then, about 70 bisbibenzyls have been reported (Sen K et al., Plants 2023, 12, 4173). The significance of these compounds in different bioactivities like antibiotic, antioxidative, antitumor, antivenomous, anti-influenza, insect antifeedant, cytotoxic, and anticancerous activities are surveyed. Among them, radulanin has been described and is now examined in the field of herbicides. Its mode-of-action has been elucidated (Thuillier S et al., Pest Manag Sci 2024, 80,:156).
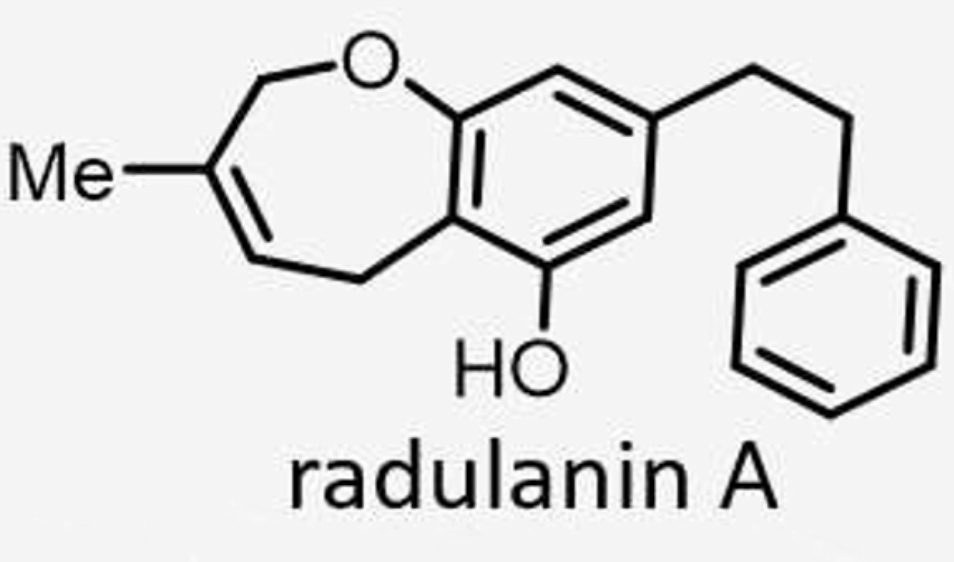
The 1,4-benzoquinone moiety linked to the sesquiterpene is a common structural feature in a large number of terpenoid compounds which have a large spectrum of biological activities. Besides avarol, several other natural quinones and hydroquinones, such as illimaquinone, nakijiquinone and bolinaquinone, are present in sponges of the order Dictyoceratida. All have cytotoxic and antiproliferative properties which offer promising opportunities for the development of new antitumor agents. A review of the cytotoxic termene quinones from marine sponges has been released by Gordaliza M (Mar Drugs 2010, 8, 2849).
Different sesquiterpene quinones were isolated from the red alga Laurencia johnstonii have been studied to find a treatment against the tropical diseases, leishmaniasis. Among isolated compounds, one has been identified with a strong leishmanicidal activity: laurequinone, more effective than the reference drug miltefosine against promastigotes (García-Davis S et al., Mar Drugs 2023, 21(6), 333).
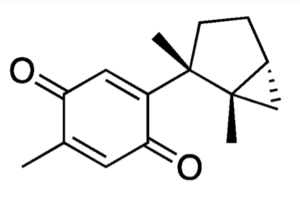 Laurequinone
Laurequinone
Isolinderalactone was shown to suppress human glioblastoma growth and angiogenic activity (Lee SY et al., Oncol Lett 2022, 24, 328). This sesquiterpene extracted from the dried root of Lindera aggregata had the ability to alleviate cellular damage induced by β-amyloid-1–42 (Aβ1–42), suggesting that it may be a potential drug candidate for the treatment of Alzheimer’s disease (Xiong L et al., J Nat Prod 2023, 86, 2718).
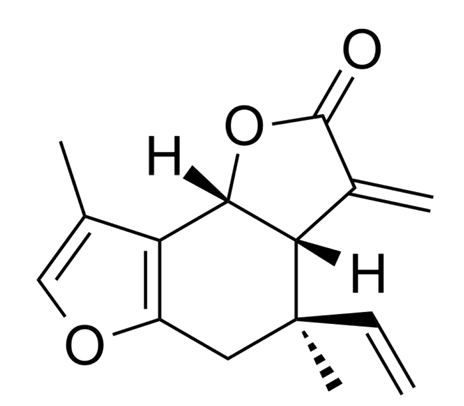
Isolinderalactone
Artemisinin and its derivatives are all sesquiterpene lactones used for the treatment of malarial and parasitic worm (helminth) infections and containing an unusual peroxide bridge. This endoperoxide 1,2,4-trioxane ring is responsible for their antimalarial properties. It was extracted from the plant Artemisia annua (sweet wormwood), an Asteracaea employed in Chinese traditional medicine. On account of the poor water solubility of artemisinin an attempt was made to improve either its water solubility or its lipid solubility. Dihydroartemisinin is a semi-synthetic antimalarial compound derived from artemisinin, and more potent than its parent compound. Beyond its antimalarial properties, it also demonstrates notable antineoplastic activities across various cancers. Recent studies have elucidated the anti-tumor effects of artemisinin derivatives (Zheng P et al., Food Biosci April 2025, 106648).
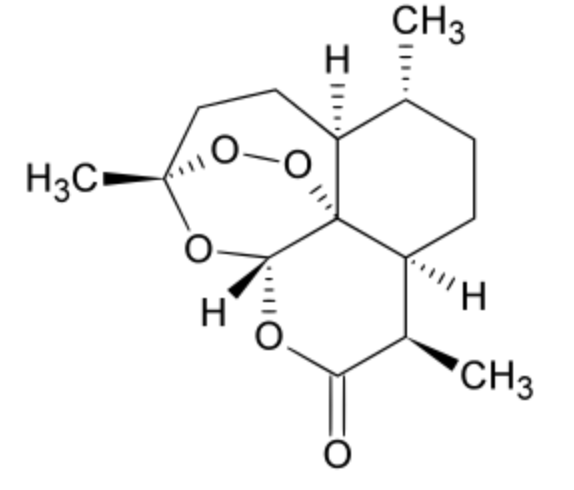 Artemisinin
Artemisinin
Matricarin is a natural product found in several different foods, such as herbal tea, german chamomile or Matricaria recutita, Asteraceae which represent the drug usually termed as German Chamomile. An allied plant source Chamaemelum nobilc, normally known as Roman Chamomile also comprises of identical components and used alike. Chamomile is the most popular ‘herbal tea’ in the United States because of its definite anti-inflammatory and antipasmodic therapeutic properties.
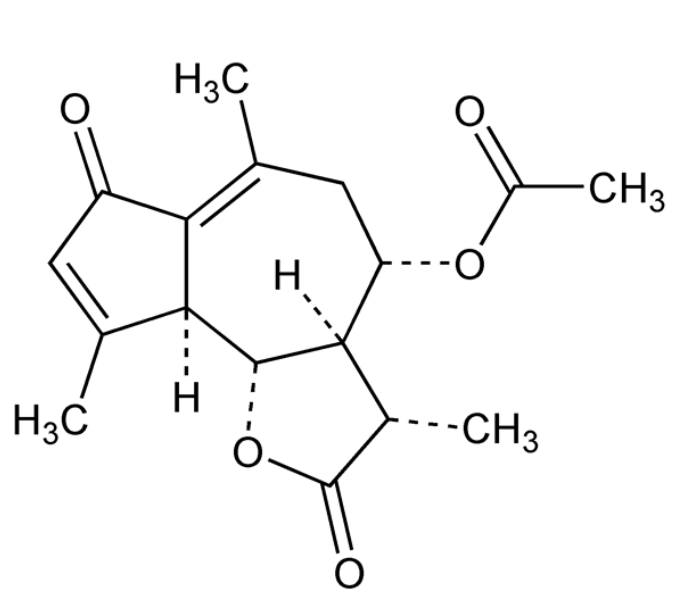 Matricarin
Matricarin
Rasmann E et al. (Nature 2005, 434, 732) discovered that insect-induced (E)-β–caryophyllene emission from maize roots attracts nematodes that prey on the attacking insect larvae (Diabrotica virfera) which are feeding on maize roots. This volatile terpene is thus highly attractive to the entomopathogenic nematode which parasitizes and kills the larvae within a few days.
The first total synthesis of caryophyllene in 1964 by the American chemist EJ Corey (Nobel prize in 1990).
(E)-β-Caryophyllene
Caryophyllene is a constituent of many essential oils, especially clove oil (flowers of Syzygium aromaticum), the essential oil of cannabis (Cannabis sativa), black peper, true cinnamon, lavender, oregano, rosemary, basil and hops. It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and a-humulene. It contributes to the spiciness of black pepper. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.
It was shown that it is also a selective agonist of cannabinoid receptor type-2 (CB2) and thus could exert significant cannabimimetic antiinflammatory effects, but it does not bind to cannabinoid receptor type 1 and thus does not induce psychomimetic effects. Experiments have proved that humulene, when adsorbed to neighbouring species foliage, could lead to associational resistance and help plants protect themselves as a result of coexistence (Himanen SJ et al., New Phytologist 2010, 186, 722).
It has various pharmacologic properties such as cardioprotection, hépatoprotection, gastroprotection, neuroprotection, néphroprotection, anti-inflammatory effects and immunomodulatory effects. Current studies are hoping to unveil even more of the therapeutic potential behind caryophyllene.
It is well known that sesquiterpenes are highly reactive chemicals and that they are very abundant among the biogenic substances emitted by the vegetation and soil microorganisms, a great part being played by β-caryophyllene. β-Caryophyllene is among the most abundant sesquiterpenes emitted by pine and citrus trees, among other agricultural plants (Duhl TR et al., Biogeosciences 2008, 5, 761). It has been demonstrated that a low proportion of caryophyllene increases the volatile organic compound yield and doubles their formation rate. Its contribution in new particle formation is expected to be a major source of cloud condensation nuclei (Dada L et al., Sci Adv 2023, 9, eadi5297).
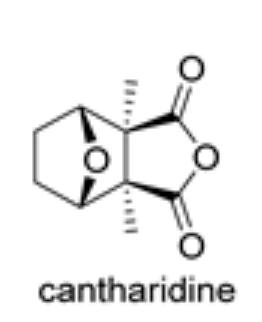
Cantharidin is the most relevant compound of the sesquiterpenoid anhydride group. It is produced in two insect families, blister beetle (Coleoptera meloidae) and false blister beetle (Coleoptera oedemeridae) (Molina Inzunza DO et al., Biomolecules 2024, 14(8), 955). There are currently more than 1500 species of cantharidin-producing beetles. Cantharidin acts as a chemical defense (vesicant) of insect producers against predatory organisms and has been used since ancient times (for more than 2000 years in China) for its biological activities. Insects containing that compound were the first insects historically used in traditional medicine since ancient times for the treatment of innumerable ailments. In Europe, it appeared in Materia Medica, a medical monograph written by Pedanios Dioskorides in 50 to 100 AD and Hippocrates prescribed cantharidin as a treatment for dropsy (Moed L et al., Arch Dermatol 2001, 137, 1357). Cantharidin and similar compounds are mainly used in dermatology for their vesicant properties in the removal of tattoos, for the treatment of plantar warts in podiatry and the treatment of the virus Molluscus contagiosum.
Three types of euryspongins (euryspongins A, B, and C) were procured from the marine sponge named Euryspongia species (Yamazaki H et al., Bioorg Med Chem Lett 2013, 23, 2151). These bioactive sesquiterpenes produced the therapeutic action by inhibiting the enzyme PTP1B, which is considered as one of the important targets in diabetes therapy (Yamazaki H et al., Bioorg. Med. Chem. Lett 2013, 23, 2151)..
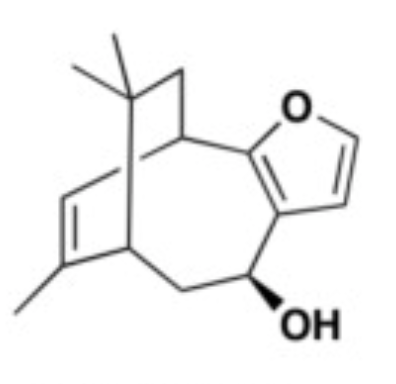 Euryspongin A
Euryspongin A
Sandalwood oil is composed of more than 50% of santalenes (exo-α-bergamotene, epi-β-santalene, β-santalene, and α-santalene) and santalols (Z-epi-β-santalol, trans-α-bergamotol, Z-β-santalol, and Z-α-santalol). Sandalwood remains among the largest and most popular plants frequently used in the perfume and cosmetics sectors and should be recognized as one of the leading medicinal plants (Review in : El Hachlafi N et al., Ind Crops Products 2024, 214, 118567). Synthetic processes have been developed to produice α-santalol and β-santalol via fermentation, mainly using Rhodobacter sphaeroides. Subcellular engineering has been explored to improve santalol production using Saccharomyces cerevisiae (Wang D et al., J Agric Food Chem 2025, 73, 20307).
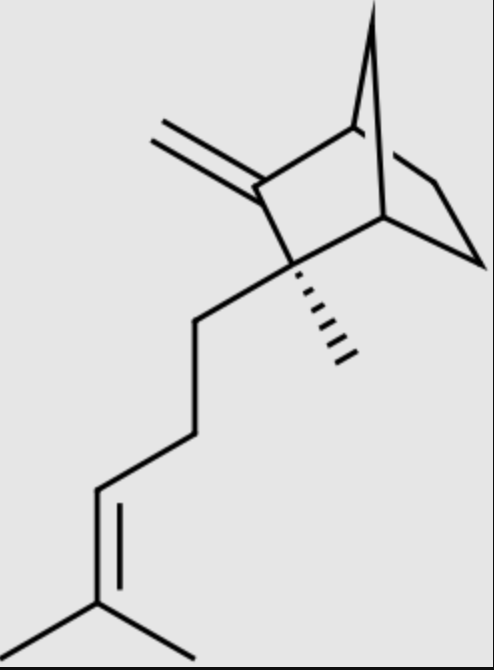 β-Santalene
β-Santalene
Patchoulol or patchouli alcohol is a sesquiterpene alcohol found in patchouli oil which is an important material in perfumery. The (−)-optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is obtained by fermentation of leaves of Pogostemon cablin, an aromatic and tender perennial herb from the mint family.
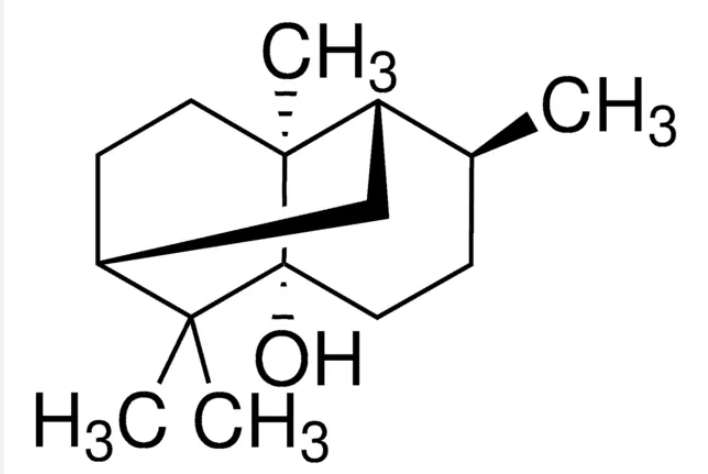 Patchoulol
Patchoulol
Trichothecenes – They constitute a large group of chemically related mycotoxins which are produced by fungi, the most important being Fusarium, All trichothecenes share the cyclic sesquiterpene structure but differ in the type of functional group attached to the carbon backbone. Each displays a core structure consisting of a six-membered ring containing a single oxygen atom, flanked by two carbon rings. This core ring contains an epoxide bridging carbons 12 and 13, as well as a double bond between carbons 9 and 10. These two functional groups are primarily responsible for trichothecenes’ ability to inhibit protein synthesis and incur general cytotoxic effects. Nivalenol belongs to the trichothecene group and is well known for its toxicity. It is mainly found in fungi of the Fusarium species. It primarily exerts its toxicity in the body by disrupting the integrity of intestinal epithelial cells, inducing oxidative stress, triggering inflammatory responses, interfering with cellular signaling pathways, inducing apoptosis, and impairing DNA repair mechanisms.
Nivalenol as well as deoxynivalenol (or vomitoxin), which are commonly found in cereals like wheat and maize, have been used as biological warfare agents in Laos and Cambodia as well as in Afghanistan. According to the World Health Organization, approximately 10% of the global population may be affected by dietary and environmental exposure to deoxynivalenol. Its male reproductive toxicity has been reported and studied in detail (Huo H et al., Food Biosci online 10 February 2026, 108456).

One Trichothecene
Copaene is a tricyclic sesquiterpene found in a number of essential oil-producing plants. It was first described in the resin-producing tropical copaiba tree, Copaifera langsdorffii. The α-copaene enantiomer is most commonly found in higher plants. It has been determined that copaene is the main volatile substance emitted by maize (Zea mays) plants damaged by the fall armyworm, Spodoptera frugiperda, which attracts Chelonus insularis (Hymenoptera: Braconidae), an egg-larval endoparasitoid that attacks the fall armyworm (Ortiz-Carreon FR et al., J Chem Ecol 2019, 45, 326).
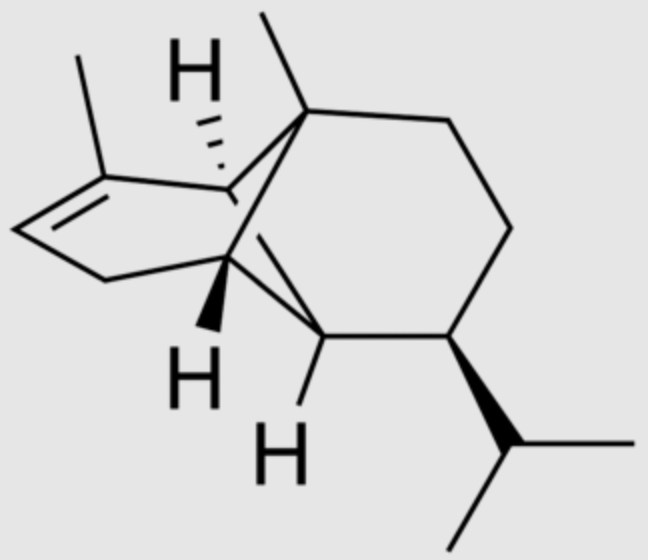 α-Copaene
α-Copaene
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire