
After the very early demonstration by Chevreul in 1823 that the fusion temperature of variously unsaturated fatty acids depends on the number of double-bonds per molecule, it is now well known that the texture of margarine may be altered through the composition of its triglyceride molecular basis. This is easily achieved by modifying the distribution of the fatty chains on the glycerol molecule via an important catalytic reaction, interesterification.
While the first published mention of the synthesis of a triglyceride (esterification of glycerol by butyric acid) was by Pelouze in 1844 (Pelouze J et al Ann Chim Phys 1844, 10, 434), the alcoholysis reaction between tristearin (and tripalmitin) and ethanol (and amylic alcohol) was described in 1852 (Duffy PJ, J Chem Soc 1852, 5, 303).
Thus, fatty acid methyl esters can be conveniently prepared by displacing the glycerol from the trigyceride molecule, in heating a mixture of triglycerides with methanol (or another alcohol). If the alcoholysis reaction is conducted at high temperature, it can be made to go in the reverse direction with the formation of glycerides from esters of the volatile alcohol. If a small proportion of glycerol is used, the migration and interchange of the fatty acid radicals lead to the formation of triglycerides of new composition. It was found that the use of glycerol was not necessary, the use of alkaline catalysts is sufficient.
It appears that Normann W (who also patented earlier the catalytic hydrogenation of fatty acids) was the first to patent the chemical interesterification of edible lipids in 1920 (German Patent 417, 215 with Firma Oelwerke Germania GmbH). Van Loon C of Anton Jurgens Company patented the industrial process of glyceride rearrangement in England in 1924 and in Netherlands in 1927, Grün A (US Patent 1, 505, 560 in 1924) and Barsky G (US Patent 2, 182, 332 in 1939) in the United States. This process was viable in the food industry since the 1940s to improve the spreadability and baking properties of lard but was largely developed in the 1970s as a hydrogenation replacement for the manufacture of margarines without trans-fatty acids. More recently, interesterification was used in the production of low calorie fat replacers (Olestra, Salatrim…)
At the beginning, fats were heated at 100-200°C with 0.1% sodium ethylate or stannium hydroxyde until the needed randomization degree was reached. Now, K glycerolate or Na methylate is used, any traces of catalyzer being removed by washing with water.
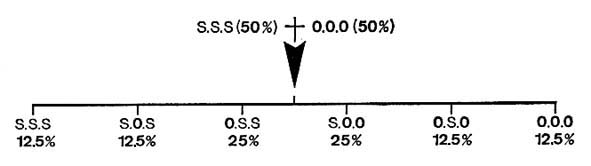
The figure below shows how are obtained several randomized heterogeneous triglycerides by interesterification of a mixture of tristearine (SSS) and triolein (OOO).
The build up of triglycerides with determined structure is realized through the use of enzymes (lipases, esterases) as transferases in 3 types of reactions :
transesterification : exchange of carbonyl groups between esters
acidolyse : exchange of carbonyl group between esters and carboxylic acids (fatty acids)
alcoholyse : exchange of alcohol between esters and alcohols.
Acidolysis is the most commonly used process to enrich vegetal oils in one component (linoleic, oleic or eicosapentaenoic acid). Lipases (e.g. from Rhizopus) are used to catalyze this reaction thus exchanging fatty acids in the 1 and 3 positions with free fatty acids in the medium. The secret of specific syntheses has been the selection of lipases with the correct specificity and chemical environment (use of aqueous/organic biphasic systems).
Advantages of enzymes over conventional chemical catalysts include substrate specificity, lower energy costs and a high quality end-product.
It is thus easy to manufacture artificial or “structured” triglycerides enriched with EPA from fish oil (beneficial to cardiovascular health), short-chain or medium-chain fatty acids to prevent obesity or hypercholesterolemia.
Structured lipids are triacylglycerols containing combinations of short-chain fatty acids, medium-chain fatty acids and long-chain fatty acids located in the same glycerol molecule. These lipids are developed to fully optimize the benefits of their fatty acid varieties in order to affect metabolic parameters such as immune function, nitrogen balance and lipid clearance from the bloodstream.
The short-chain fatty acids (C2-C4) may be acetic, propionic, butyric or a combination of all three, while the long-chain fatty acid (C16-C22) is derived from hydrogenated vegetal or animal oils. Randomized fats have diverse applications, both in the food industry and in clinical applications. Interesterified fats and oils differ from their native products in their melting and crystallisation properties since that process confers new rheological properties to the products. In clinical applications, randomization provides energy-rich substrates for parenteral, enteral and infant feeding which are well-absorbed. Industries have also made products with low energy and fat content (fat replacer).
Commercial products examples of structured lipids
Caprenin
Caprenin is a common name for cap-rocaprylobehenin, a structured lipid containing C8:0, C10:0, and C22:0 fatty acids esterified to glycerol moiety. It is manufactured by Procter & Gamble’s (Cincinnati, Ohio, U.S.A.) from coconut, palm kernel, and rapeseed oils by a chemical trans-esterification process. Thee MCFAs are obtained from the coconut oil and the LCFAs from rapeseed oil. Because C22:0 is only partially absorbed and capric and caprylic acids are more readily metabolized than other longer chain fatty acids, caprenin provides only 5 kcal/g.
Benefat
Benefat (Danisco trade name for Salatrim) is the most known blend of structured triglycerides being recognized as safe (GPAS) by the US FDA. It is proposed to consumers to manage their energy intakes without sacrificing desirable taste ans texture and interfering with the absorption of fat soluble vitamins. Salatrim is made primarily of 18:0 (50%) and 16:0 (6.6%), combined with the short-chain organic acids : acetic (C2:0, 21.7%) and butyric (C4:0, 2.6%) acids. These short-chain compounds may be treated nutritionally as carbohydrates. Thus, replacing 10g of fat with an equal amount of Salatrim would reduce the amount of actual fat available to 5.5g.
Medium-chain triglycerides containing caprylic, capric and lauric acids are manufactured as nutritional supplements, carriers for flavorings and colorings, or release agents for baked goods.
Neobee
Neobee is another caloric reduced fat, it is composed of capric and caprylic acids and produced by Stepan Company (Maywood, N.J., U.S.A.). This class of specialty lipids includes different products. For example, Neobee 1053 and Neobee M-5 contain both capric and caprylic acids, while Neobee 1095 is made up of only capric acid. Neobee 1095 is a solid product. Therefore, this product may be suitable in certain applications which require solid fats. Neobee 1814 is an MCT derivative made by interesterification of MCT with butter oil; it contains half of the long-chain saturated fatty acids found in conventional butter oil and is suitable to replace butter oil in a variety of applications. Neobee 1814 may serve as a flavor carrier and functions as a textural component for low-fat food products.
Interesterification is used by the food industry to alter the melting and crystallization characteristics of fats to change certain functionality, such as spreadability or a particular melting point. Interesterification influences the physical properties of fats due to differences in the individual melting and crystalline properties of molecular species. The overall effect is the generation of a hard fat from a soft fat.
As well as achieving suitable melting properties, interesterification also optimises crystallisation behaviour to generate more stable crystalline forms. Saturated triacylglycerols can exist in more than one crystalline form (polymorphs), which results in different patterns of molecular packing in the crystals and multiple melting points. The three basic polymorphs are referred to as a, b‘ andb. The a form is the least stable with the lowest melting point, and b the most stable with the highest melting point. For example, the melting point of the a, b‘ and b polymorphic forms of the species stearic/oleic/stearic (SOS) are 22.4, 36.5 and 41.7° C, respectively. During interesterification, the b‘ form is commonly generated, thus improving the stability and granularity of the fat. For example, the proportion of solid fat at body temperature (37° C) in the native cocoa butter and randomly interesterified cocoa butter is 1 and 37% respectively (Berry SEE, Nutr Res Rev 2009, 22,3).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire