
LONG-CHAIN ALDEHYDES
Long-chain aldehydes occur in free form as in vegetal oils, essential oils, and insect extracts (pheromones). Most frequently, the lipidologist will find them in the form of vinyl ether (alk-1-enyl ether) in plasmalogen analogs of glycerides and phospholipids.
A review of mass spectrometry techniques for fatty aldehydes separation and determination may be consulted (Berdyshev EV, Biochim Biophys Acta 2011, 1811, 680).
Separation
When aldehydes must be isolated on a preparative level lipid samples in hexane are filtered through a silica gel column. After a preliminary wash of hexane, aldehydes are eluted with hexane/ether (98.5/1.5). Waxes, if present, are also found in that fraction.
Isolation on a SPE cartridge : The silica cartridge is washed with 5 ml hexane. The lipid sample (about 50 mg for 1 g silica) mixed with 2 drops of Sudan I solution (1 mg/ml hexane/ether, 99/1) is applied to the column. After draining, the column is eluted with 7 ml of hexane/toluene (85/15, v/v) to eliminate hydrocarbons. Then, the column is eluted with about 10 ml of the same mixture until the stain reaches the bottom of the column. This fraction is evaporated and re-dissolved in a small volume of heptane until direct GC analysis (Perez-Camino MC et al., J Chromatogr A 2003, 963, 283).
Isolation by TLC : These components are most readily obtained by TLC on silica gel plates using petroleum ether/diethyl ether (90/10, v/v). Fatty aldehydes migrate at a Rf of about 0.55 just below fatty acid methyl esters taken as references. Standard aldehyde are also available commercially (hexa- and octadecanol from Sigma).
If necessary, long-chain aldehydes may be prepared by oxidation of the corresponding alcohols using pyridinium dichromate (see below).
How to detect aldehydes on TLC plates ?
Aldehydes in free or bound (vinyl ether, dimethyl acetal derivatives) form react with 2,4-nitrophenylhydrazine to form nitrophenylhydrazones which have a strong orange color.
Spray TLC plates in a fume hood with a solution of 0.4 % 2,4-dinitrophenylhydrazine (w/v) in 2M HCl. Yellow orange spots are visible on warming (at 100°C) the plates.
The lipid extract is dissolved in 3.5 ml of chloroform/methanol (2/1) containing 30 µl acetic acid. 5 mg of 2,4-dinitrophenylhydrazine are added and the solution is allowed to stand 1 h at room temperature in darkness (or 20 min at 60°C in a closed tube). At the end of the reaction time, wash with 0.7 ml of saline solution (9g NaCl/l water), vortex and centrifuge. The lower phase is evaporated, the residue dissolved in 2 ml hexane and washed twice with 2 ml of 50 % ethanol. Measure the absorbance of an aliquot of the hexane phase at 360 nm against a reagent blank carried through the procedure. Calibrate the procedure with a pure fatty aldehyde solution (0.1-1 µmole per tube). An estimation can be made knowing that the molar extinction coefficient of the hydrazones is about 24 000.
Gas liquid chromatography
A – Fatty aldehydes or plasmalogens can be adequately quantified by GLC after conversion to dimethylacetal derivatives by reaction with BF3/methanol reagent used also for fatty acid methylation.
When fatty acids are present (in plasmalogen analysis), the two classes can be separated by TLC, but the most common dimethylacetals (16:0, 16:1, 18:0 and 18:1) present in plasmalogens are easily separated by GLC on a capillary column. They are separated according to chain-length and number of double bonds as the analogous fatty acid methyl esters. Their retention times on a polar column (Carbowax) are shorter than the corresponding fatty acid methyl esters: 16:0 and 16:1 dimethylacetals have retention time between that of 15:0 and 16:0 methyl esters and 18:0 and 18:1 dimethylacetals between that of 17:0 and 18:0 methyl esters.
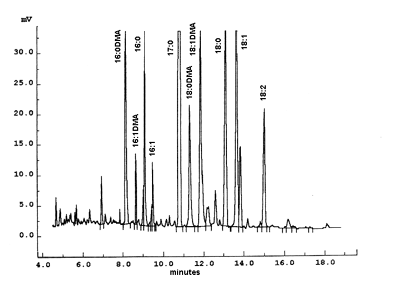
The figure shows the portion of a chromatogram corresponding to a sample of phosphatidylethanolamine purified from mammalian erythrocytes and transmethylated with BF3/methanol. Heptadecanoic acid (17:0) was added as an internal standard. The analysis was performed with a carbowax fused silica 30 m x 0.25 mm ID capillary column at 240°C. Each peak can be characterized by a relative retention time: ratio of its retention time by the retention time of a reference compound (usually 18:0).
To identify properly dimethylacetals in plasmalogens, it is recommended to transmethylate a sample of phosphatidylethanolamine from bovine brain containing approximately 60% plasmalogen (Sigma P9137) and to make comparisons with a phosphatidylcholine sample (giving only some traces of DMA). The relative position of each one of the most frequent DMA obtained from animal extracts can be compared with those shown in the chromatogram.
How to liberate aldehydes from plasmalogens ?
The plasmalogens are dissolved in 2 ml of diethyl ether and 1 ml of concentrated HCl is added. After 1 min vortexing and short centrifugation, the upper ether layer is removed and the aqueous phase is washed again with the same volume of ether and hexane. All the organic phases are washed with water (2 ml) before its evaporation. The free aldehydes may be purified by TLC on silica gel layers (solvent system: hexane/diethyl ether, 90/10, v/v), if necessary, they are transformed into dimethylacetals and analyzed by GLC.
Gas chromatography analysis of native long-chain aldehydes
Underivatized long-chain aldehydes are separated on a Restek 65TG capillary column (coated with 35% dimethyl and 65% diphenylpolysiloxane).
Oven temperature : 90°C for 0.2 min, increase at 20°C/min up to 160°C then increase at 4°C/min to 260°C. If waxes are present, the final temperature must be set at 350°C (Perez-Camino MC et al., J Chromatogr A 2003, 983, 283).
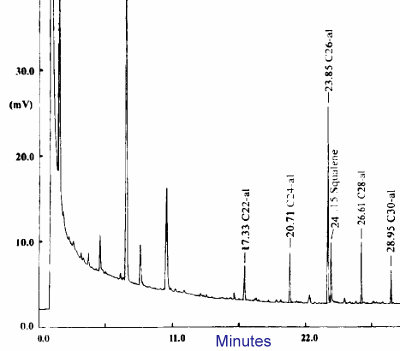
GC analysis of aldehydes with 22 up to 30 carbon atoms
The quantification of pentafluorobenzyl oxime derivatives of long chain aldehydes by GC-MS has been described (Brahmbhatt VV et al., Lipids 2008, 43, 275). The limit of detection was determined to be 0.5 pmol with a linearity over two orders of magnitude.
A facile method for the detection of aldehydes in oxidized lipids using solid-phase microextraction fiber and gas chromatography has been proposed (Arato S et al., J Oleo Sci 2009, 58, 58, 17).
Preparation of long-chain aldehydes
The corresponding alcohols are oxidized to aldehydes using pryridinium dichromate in CH2Cl2 (Bijoy P et al., Synth Comm 1993, 23, 2701).
To a stirred solution of pyridinium dichromate (Aldrich) (5 mmol) and celite (2 g) in dry methylene dichloride (12 ml) is added a solution of the alcohol (1 mmol) in 4 ml of the same solvent. The mixture is stirred at 25°C for 24 hours. Diethyl ether (40 ml) is added and the reaction mixture is filtered through celite and washed with ether (2×20 ml). The combined filtrate is evaporated and re-dissolved in a small amount of ether and purified by filtering through a silica gel column.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire