
GLOBAL METHODS
Numerous methods have been described for the quantitative measurement of fatty acids in biological materials. They can be classified into four categories:
![]()
1. Chemical titration methods
Fatty acid concentrations equal or higher than 1 mM may be easily determined by titrimetry even in the presence of other lipids. Titrimetry was classically used to determine the acid value (free fatty content) of vegetable oils and fats. This acid value is defined as the number of mg of KOH required to neutralize the fatty acids contained in 1 g of the fat. It is very easy to express the results in other units as mg fatty acids per g of sample or mmoles per Kg.
Macro-method
Reagents:
solvent mixture (95% ethanol/diethyl ether, 1/1, v/v). 0.1 M KOH in ethanol accurately standardized with 0.1 M HCl (pure ethanol may be also used if aqueous samples are analyzed), 1 % phenolphthalein in 95% ethanol.
Procedure:
Weigh 0.1 to 10 g of oil or fat (according to the expected acid value) in glass vial and dissolve in at least 50 ml of the solvent mixture (if necessary by gentle heating).
Titrate, with shaking, with the KOH solution (in a 25 ml burette graduated in 0.1 ml) to the end point of the indicator (5 drops of indicator), the pink color persisting for at least 10 s.
The acid value is calculated by the formula: 56.1 x N x V / M
where V is the number of ml of KOH solution used and N his exact normality, M is the mass in g of the sample.
Other expressions can be easily calculated (concentration of fatty acids or their weight, considering an average molecular weight of 282).
A non-aqueous flow injection titrimetric method has been described for the determination of free fatty acids in vegetal oil samples even with a background colour (Saad B et al., Food Chem 2007, 102, 1407).
Micro-methods
1 – When small amounts of fatty acids (less than 1 mmole per sample) must be determined it is convenient to use a titrimetric micro-method. Among the first reported method the most accurate and rapid is that of Dole where details on the extraction of non-esterified fatty acids from plasma are also given (Dole VP, J Clin Invest 1956, 35, 150).
We give below the part of the Dole’s paper devoted to the accurate extraction and titration of fatty acids (down to about 20 nmoles per sample). When lipids are extracted into a heptane phase, the procedure can be considered specific for fatty acids, at least with respect to phospholipids or acidic sulfolipids.
Extraction mixture: isopropyl alcohol 40 parts; heptane 10 parts; 1 N H2SO4 1 part (all solvents redistilled).
Titration mixture: 0.01 per cent thymol blue and 90 per cent ethanol in water, made by dilution of a stock 0.1 per cent thymol blue in water with 9 parts of redistilled ethanol. The titratable acidity of ethanol slowly increases over a period of several days, but is easily reduced to a minimum value by addition of alkali from the burette. A slight acidity, equivalent to about 2 microliters of 0..18 N NaOH per cc. of titration mixture, is required to expel carbon dioxide from the system during titration. Alkali: approximately 0.018 N NaOH, made by 1/1000 dilution of saturated NaOH with carbon dioxide-free distilled water. The alkali, protected with a soda lime column, is stored in a small reservoir mounted above a 0.100 cc Rehberg burette (equivalent to a micro-syringe). Fresh alkali, prepared each day of analysis, is calibrated by extraction and titration of standards of recrystallized palmitic acid in heptane.
As soon as possible after separation of plasma, 5 cc. of extraction mixture is added to 1 cc. of plasma in a glass-stoppered tube and shaken vigorously for a moment. After standing 10 minutes or longer the system is divides into two phases by mixing into it an additional 2 cc. of heptane and 3 cc. of water. The phases should separate rapidly without centrifugation, forming a sharp interface. A 3 cc. aliquot of upper phase is transferred to a 15-cc. conical centrifuge tube containing 1 cc of titration mixture, and titrated with the alkali. Nitrogen, delivered to the bottom of the tube with a fine glass capillary, expels carbon dioxide from the sample and keeps the two phases mixed during titration. As the green-yellow endpoint is approached the gas stream is interrupted from time to time for examination of the indicator color in the alcoholic phase. Good lighting, such as that given by a fluorescent light placed just above and in front of the tube, has been found helpful in reading the endpoint.
In this important paper, Dole very convincingly demonstrated that the acidity “resides in albumin-bound fatty acids”. Furthermore, he determined that only 5 per cent of the fatty acids remained free in solution.
2 – An improved titrimetric method for determining the free fatty acid (FFA) content in various lipids, even when the samples are highly colored or rancid, was described (Ke PJ et al., Anal Chim Acta 1978, 99, 387). This method is largely used to determine routinely the quality index for fish products.
Reagents : An aqueous solution (0.5%) of m-cresol purple was used as the indicator. Aqueous 0.05M NaOH was the titrant. Chloroform.
Procedure : The lipid sample (about 1g) was accurately weighed and dropped into a 250 ml Erlenmeyer flask containing 50 ml of chloroform. After 5 drops of m-cresol indicator has been added, the solution was titrated to the purple end-point with aqueous 0.05M NaOH. A blank titration should be made daily.
The FFA may be calculated as FFA mmol per gram of sample = 1000 NV/W.
N, V, an W denote the molarity of the titrant, the volume (ml) of titrant, and weight (g) of sample, respectively.
2. Thermometric titration method (non-aqueous media)
This method permits rapid, accurate, automated analysis of the free fatty acid content of oils or fats with unprecedented precision and accuracy. The method uses a technique which may be referred to as Catalyzed Endpoint Thermometric Titrimetry or CETT (Smith TK, J Am Oil Chem Soc 2003, 80, 21-24). The basic titration resembles the current standard method where free fatty acids are titrated with a standard solution of KOH in iso-propanol. The first excess of hydroxyl ion after neutralization of fatty acids in a measured sample of lipids catalyzes a strong exothermic reaction between components of the solvent mixture (acetone/chloroform, 25/2, v/v) dissolving the fat or oil.
A simple thermistor is used to sense the change in solution temperature which signals the sharp and unequivocal titration endpoint. The modern thermometric titration system (MULTITRATOR) employs powerful algorithms to optimally condition the temperature signal and permit the computation of derivatives to accurately locate endpoints.
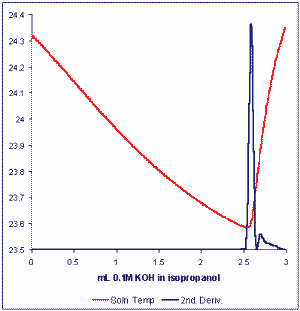
The time taken for analysis of free fatty acids by this method is typically 1-3 minutes and the Multitrator titration system allows for a full automation. Typical analytical precisions obtained have been 0.001 % fatty acids (as oleic acid) for vegetable oils. The same method may also see application for the determination of Total Acid Number (TAN) in lubricating oils.
The thermometric probe requires no maintenance or calibration, and titrations are carried out under normal laboratory conditions in polypropylene beakers.
3. Measurement of metal-fatty acid complexes
The ability of fatty acid to form complexes with some metals (Cu, Co) and to be detectable by spectrophotometry was formerly used. These methods require long analysis times and show poor sensitivity. An improved sensitivity was reported using the radiochemical assay of the complex of fatty acids with 60Co (Ho RJ et al., Anal Biochem 1969, 31, 426-436).
Reagents
Saturated solution of sodium sulfate, saturated solution of potassium sulfate, cobalt nitrate, acetic acid, triethanolamine, chloroform, heptane, 60Co nitrate (2 mC/10 ml Co nitrate solution).
Reagent solution: prepare before use a mixture of 10 vol. of Co nitrate solution (2 mmoles in 100 ml of saturated Na sulfate containing 0.8 ml acetic acid), 9 vol. of saturated K sulfate solution, and 1 vol. triethanolamine.
Procedure
To a small test tube are added 50 ml heptane solution of fatty acids varying from 0.1 to 200 nmoles, 100 ml chloroform/heptane mixture (4/1, v/v), and 10 ml of reagent solution containing 100 nmoles Co nitrate, and 1 to 7 x 105 cpm 60Co. The tube is stoppered, vortexed for 30 s, and centrifuged for 20 min at low speed. A 100 ml aliquot of the upper phase is collected for radioactivity counting. Counts are corrected for background and the amount of fatty acids is calculated from the counts obtained from standard fatty acid solutions.
Alternative methods
A simple colorimetric micro-determination of free fatty acids in plasma using microplate readers was proposed (Tinnikov AA et al., Clin Chim Acta 1999, 281, 159). This method based on the formation of fatty acid-copper complexes allows the precise determination of fatty acid concentration in 10 ml of numerous samples of plasma. The reported procedure is a valuable modification of the procedure of Laurell S et al. (Clin Chim Acta 1967, 16, 57).
Duncombe used a colour reagent, sodium diethyldithiocarbamate, to react with the copper solution in the organic phase containing fatty acids, thus developing a yellow colour, which improved the sensitivity (Duncombe W, Biochem J 1963, 88, 7). This improved method was applied for the measurement of the lipids in microalgae by converting lipids to fatty acids through saponification (Wawrik B et al., J Microbiol Methods 2010, 80, 262).
An adaptation of the Lowry method (Lowry RR et al., JAOCS 1976, 53, 473) using cupric acetate-pyridine reagent has also been proposed for the analysis of free fatty acids in fish meat (Bernardez M et al., J Agric Food Chem 2005, 53, 1903).
After extraction of meat lipid according to the classical Folch method, the whole pool of free fatty acids may be estimated according a modification of the Lowry RR et al. method. The dry lipid extract is dissolved in 3 ml cyclohexane and mixed with 1ml of cupric acetate-pyridine reagent. After agitation and centrifugation, the upper layer was read at 715 nm and the free fatty acid concentration is calculated as micromolar oleic acid based on a standard curve.
It was discovered that the fatty acid–copper complex in the organic phase can be directly detected by spectrometric analysis in the ultraviolet region (maximum absorbance at 260 nm) without any colour developer (Chen Y et al., Anal Chim Acta 2012, 724, 67). The results showed that this method could be used to analyze low levels of lipids in the range of nanomoles from as little as 1 mL of microalgal culture.
4. Enzymatic methods
The introduction of acyl-CoA synthetase enabled to quantify fatty acids in biological fluid in coupling the formation of activated fatty acids with three enzymatic reactions which lead to a fluorimetric detection of NADH consumption (Jebens E et al. Scand J Clin Lab Invest 1992, 52, 717).
The method was described for the determination of plasma free fatty acids but may be adapted to small lipid extracts dissolved in Triton X-100 solution.
This method is very simple and inexpensive and well suited for automation. The presence of up to 10 nmoles fatty acids in the sample tube is accurately determined with minimum interference from other compounds.
5. Method using a fatty acid binding protein
This method is particularly useful for the measurement of very low concentrations of fatty acids such as those found for the free fatty acid pool in biological fluids.
Measurement of the true free fatty acid amounts
Fatty acids produced for example by adipose cells are transported within the blood in close association with serum albumin (Spector AA, J Lipid Res 1975, 16, 165). In equilibrium with that bound fatty acids, a small fraction exists dissociated from albumin in monomeric form in the aqueous phase, they are the free fatty acids (FFA). They were shown to play various roles in several physiological events (cellular energetics, ion transport…) and in diseases (cancer, diabetes, ischemia…).
An estimation of FFA levels is possible using measured equilibrium constants and the ratio of total fatty acids to albumin (Spector AA et al., Biochemistry 1971, 10, 3229).
A direct measurement of FFA in aqueous solutions is now possible, using a fluorescent probe ADIFAB, composed of a fatty acid binding protein derivatized with the fluorescent molecule acrylodan (Richieri GV et al. Biochemistry 1993, 32, 7574). When excited at 385 nm, the ratio of light emission at 505/432 nm can be converted to accurate FFA concentrations. This measurement can be used with serum (Richieri GV et al., J Lipid Res 1995, 36, 229) and also to monitor FFA production by cells or enzyme activities (Richieri GV et al., Anal Biochem 1995, 229, 256).
The method requires only ADIFAB (Molecular Probes, A-3880) as specific reagent and FFA concentrations in the range 1 nM to 20 mM may be determined with 10-20 ml of serum.
As the dissociation constants depend on the fatty acid molecular species, absolute amounts of FFA in biological fluids are estimated using a weighed average of the Kd for the different fatty acids in these fluids. Thus, the previous knowledge of the fatty acid profile is required for precise determinations.
Detailed information on the ADIFAB method can be found in the 1999 paper of Richieri GV et al. (Mol Cell Biochem 1999, 192, 87-94)
A Fourier-transform infrared spectroscopic technique has been developed for the non-invasive measurement of saturated and unsaturated fatty acid compositions in human mucosa (Yoshida S, Lipid Technol 2008, 20, 184). According to authors, this technique may provide a great advancement in the fields of food science, preventive medicine and epidemiology.
The determination of linolenic acid and linoleic acid iin edible oils was found to be possible using an improved near-infrared spectrometry (Wu D et al., Anal Chim cta 2009, 634, 166).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire