
SEPARATION OF FATTY ACIDS
BY HPLC
NORMAL FATTY ACIDS
Analysis of common fatty acids (with one straight chain and one acid group) is usually carried out by GLC but in special cases it may be necessary to process HPLC separations. The greatest value of HPLC is for volatile components (short chain fatty acids), for preparative scale separations or for studying isotopically labelled fatty acids. A simple and rapid method for determination of short-chain fatty acids by HPLC with ultraviolet detection has been reported (Stein J et al., J Chromatogr 1992, 576, 53). For some samples, these short-chain fatty acids may be previously concentrated by ultrafiltration (Chen HM et al., Clin Chem 1989, 35, 74). A headspace solid-phase microextraction procedure for the determination of free volatile fatty acids in waste waters has been reported (Abalos M et al., J Chromatogr A 2000, 873, 107).
Positional and conformational isomers are more easily separated by HPLC than GLC. All kinds of detectors may be used but separations of derivatized fatty acids are usually monitored with UV spectrophotometer or by fluorimetry.
Sometimes, fatty acids are separated without any derivatization either for quantitative estimation or for preparative purposes. A reversed-phased HPLC separation of underivatized fatty acids from oils and animal tissues was proposed after low temperature saponification (Nishiyama-Naruke A et al., Anal Lett 1998, 31, 2565). A simple HPLC system allowing the separation of short, medium, and long chain fatty acids has been described (Kroumova AB et al., Anal Biochem 1995, 225, 270). A more sophisticated and precise method combining HPLC and mass spectrometry was developed to measure short-chain fatty acids in blood (Van Eijk H et al., J Chromatogr B 2009, 877, 719). A precise and facile analysis of short-chain fatty acids using 4-nitrophenol as derivatization reagent has been proposed (Schiffels J et al., J Chromatogr A 2011, 1218, 5848).
Efficient purification and analysis procedures of polyunsaturated methyl esters have been described using reversed-phase HPLC and light-scattering detection (Mansour MP, J Chromatogr A, 2005, 1097, 54). A similar method has also been developed for the separation and quantitative analysis of fatty acid methyl esters in three vegetal oils (soybean, rice bran, pumpkin seed), response factors being accurately determined (Bravi E et al., J Chromatogr A 2006, 1134, 210). A HPLC method with an evaporative light-scattering detector has been developed for the separation and quantitative analysis of four underivatized long chain fatty acids present in vegetable oils (camellia oil, olive oil, Brucea javanica oil and sesame oil) (Guo H et al. JAOCS 2012, 89, 183).
Application notes with details on the detection of free fatty acids and ethyl esters with a light scattering device (Corona Charged aerosol detector) may be found on the manufacturer web site1 and web site2.
A pertinent overview of the application of reversed-phase HPLC for the separation of polyunsaturated fatty acids may be found in a paper by Dr Rao MS et al. (J Chromatogr Sci 1995, 33, 9-21).
A very sensitive fluorescence method for the direct determination of free fatty acids was proposed using the reagent DBD-PZ (Tokyo Chemical Industry Co, Product N° A5555) (Ueno Y et al., Chem Pharm Bull 1999, 47, 1375). A new BODIPY-based carboxyl-reactive fluorescent labeling reagent, TMBB-EDAN has been developed for the sensitive fluorimetric determination of FAs with HPLC (Wang FH et al., J Chromatogr A 2013, 1291, 84). The derivatization of TMBB-EDAN with fatty acids can be performed at room temperature. The detection limits range from 0.2 to 0.4 nM, which are lower than most of the derivatization-based HPLC methods for fatty acids.
The coupling of HPLC on a normal phase coupled with an ozonolysis reactor and a mass spectrometer has beeb used for the direct determination of double bond position in fatty acid mixtures (Sun C et al., Anal Chim Acta 2013, 762, 68).
Preparation of UV absorbing derivatives
The most frequently formed UV derivatives are phenacyl esters (Durst HD et al Anal Chem 1975, 47, 1797). They are the most frequently used and are easy to prepare for those who are attempting to use this technique for the first time.
The procedure using 4-bromophenacyl bromide can be recommended.
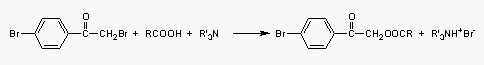
Alkaline conditions may be generated with triethylamine (prepare a 0.2 mM solution in dry acetone) but also with sodium carbonate or potassium hydroxide as catalysts.
Reagents:
4-bromophenacyl bromide, 18-crown-6 ether, 50mM potassium hydroxide (w/v) in methanol. Acetonitrile.
Procedure:
– Dissolve a sample of free fatty acids in methanol and neutralize with the KOH solution (with the help of phenolphthalein as an indicator) or triethylamine. Evaporate the mixture under nitrogen.
– Add to the residue 0.1 ml of 2mM 18-crown-6 in acetonitrile and 0.1 ml 4-bromophenacyl bromide. Heat the mixture at 80°C for 15 min in mixing gently several times. Cool the vial, dilute to a larger volume with acetonitrile to adapt the response to the fatty acid concentration.
The HPLC separation is made using a C18 column and a gradient of acetonitrile in acetonitrile/water (1/1, v/v) at 1ml/min. UV detection was set at 242 nm. The gradient shape is adapted to the fatty acid mixture and the column type but it can be recommended at the beginning to make a linear gradient portion for 60 min followed by an isocratic step (at about 80% acetonitrile) for 30-40 min. Then the gradient shape is modified according to the interest in specific fatty acids and the complexity of the sample.
An example of separation of fatty acids as p-bromophenylesters is given below.
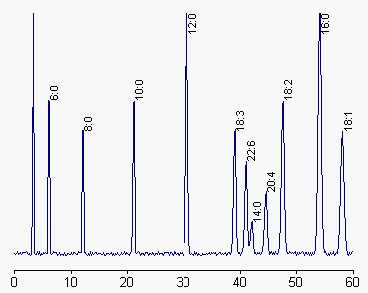
A rapid and sensitive method adapted for the quantification of free fatty acids in human plasma using phenacyl bromide esters has been proposed by Mehta A et al. (J Chromatogr B 1998, 719, 9).
An improved method of HPLC separation of biologically important fatty acids was described using a derivatization with 2-dinitrophenylhydrazine HCl (Miwa H et al., J Chromatogr 1986, 351, 275).The separation was achieved within 15 min and the detection limits ranged from 400 fmol to 1 pmol and from 100 to 200 fmol per injection with visible and ultraviolet detection, respectively. A direct derivatization procedure without commonly used isolation steps was used to analyze free serum fatty acids (Miwa H et al., J Chromatogr 1987, 416, 237).
This derivatization of fatty acids, combined with silver ion HPLC, was also successfully used to separate numerous isomers of oleic (18:1n-9) and linoleic acid (18:2n-6) (Nikolova-Damyanova B et al., J Chromatogr 1992, 609, 133).
Preparation of fluorescent derivatives
In order to increase the sensitivity and selectivity of detection a number of fluorescent derivatives have been prepared (Review: Christie WW, High-performance liquid chromatography and lipids, Pergamon Press, 1987; Marini D in Food analysis by HPLC, Nollet LMC ed, Marcel Dekker, 1992, 169). A simple derivatization method for a very sensitive determination of short and long-chain fatty acids was described by You J et al. (Anal Chim Acta 2001, 436, 163). The proposed derivatization agent for fluorescence detection by HPLC is 9-(2-hydroxyethyl)-carbazole (to be previously synthesized as not commercially available). The detection limits are said to be at 45-68 fmol level for C14-C20 fatty acids and even lower for shorter acids.
Among several labels, we have chosen bromomethylmethoxycoumarin (Br-MMC) as it is available commercially and yields strongly fluorescent compounds after a simple reaction procedure.
Proposed by Wolf et al (J Chromatogr 1988, 436, 437), the procedure described below is adapted to very low levels (ng range) of free fatty acids as well as acylated fatty acids liberated by saponification.
Reagents
Solution 1: 5 mg of Br-MMC dissolved in 5 ml dry acetonitrile,
Solution 2: 13 mg of 18-crown-6 in 5 ml dry acetonitrile,
Keep these solutions at -20°C.
Working solution: 40µl of solution 1 and 2 µl solution 2 are added to 2 ml dry acetonitrile before the derivatization.
Potassium carbonate (kept dry).
Procedure:
Derivatization: 1-2 µg fatty acids and some ng of internal standard (C17:0) dissolved in a little volume of dichloromethane are pipetted in a small amber-glass tube. After evaporation under nitrogen, add 100µl of working solution and 2-4 mg potassium carbonate. After a rapid sonication, warm 15 min at 60°C.
After cooling, filter the solution (0.22 µm nylon filter).
HPLC: use a C18 column (250 x 4 mm), a pump with a binary gradient device and a fluorimeter (Exc: 325 nm, Em: 398 nm); Eluent A: acetonitrile/water (70/30, v/v), eluent B: acetonitrile. Volume injected: 20µl.
The column is eluted with acetonitrile/water in the proportion 70/30 (v/v) initially and changed to 95.5/4.5 at 24 min for 10 min, at a flow rate of 1 ml/min.
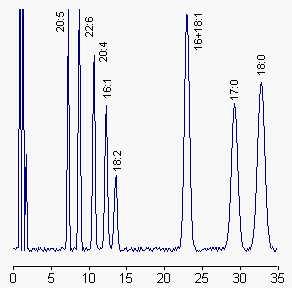
The shape of the gradient may be modified according to the fatty acids of interest.
Comments : This procedure enables the easy determination of the content and profile of free fatty acids in total lipid extracts. An example of this approach may be found in the work of Sato T et al. (JAOCS 1995, 72, 1211) using 9-anthrylmethyl esters (esterification of fatty acids with ADAM) to determine by fluorometry free fatty acids in marine phytoplankton. A one-step method for preparation of 9-anthrylmethyl esters from triacylglycerols has been described (Ando Y et al., Lipids 2007, 42, 955).
Another fluorescent reagent (NOEPES) was used for the determination of long-chain fatty acids in human plasma (Chung TC et al., Anal Chim Acta 2008, 611, 113). This method was shown to be practical and effective for the diagnosis of adrenoleukodystrophy.
A simple, stable and sensitive method for the simultaneous determination of free fatty acids from edible oils using 2-(11H-benzo[a]carbazol-11-yl)- ethyl-4-methylbenzenesulphonate (BCETS) as labelling reagent with fluorescence detection has been reported (Li G et al., Food Chem 2011, 125, 1365). Free fatty acids are derivatized by BCETS and separated on a reversed-phase column with a gradient elution.
TRANS AND CONJUGATED FATTY ACIDS
Trans fatty acids are formed by industrial and biological hydrogenation of unsaturated fatty acids. They are found in milk, butter as well as in margarines and shortenings. Here, trans-18:1 isomers predominate (the n-9 isomer, elaidic acid, being the most important). A variety of trans isomers of linoleic acid (18:2n-6) (CLA) which are formed by biohydrogenation in the rumen are found in milk and milk products and in processed foods. Commercial CLA is produced by alkaline isomerization of linoleic acid (sunflower seed oil).
A number of methods have been devised for the separation of trans isomers of unsaturated fatty acids.
In biological samples, it is preferable to fractionate the total fatty acid pool by silver ion chromatography on silica gel plates or small columns. Fatty acids obtained by saponification of acylated lipids are more conveniently fractionated in the free form and are derivatized as previously described before HPLC.
The direct estimation of the total amount of trans oleic acid was shown to be possible using a simple gas-liquid chromatographic class separation with a standard process (Thompson RH, J Chromatogr Sci 1997, 35, 536).
Furthermore, the separation of a variety of geometrical isomers of oleic and linoleic acids may be done by HPLC on reversed-phase columns. HPLC on reversed phase is able to resolve easily the four possible isomers of linoleic acid into three fractions.
Procedure:
The TLC fractions containing dienes and monoenes (delimited from standard spots of elaidic and linoleic acid) are derivatized with BrMMC as previously described and chromatographed on a C18 column with acetonitrile/water (80/20, v/v) as mobile phase at 2 ml/min. UV detection was set at 242 nm. C17:0 was added before derivatization as an internal standard.
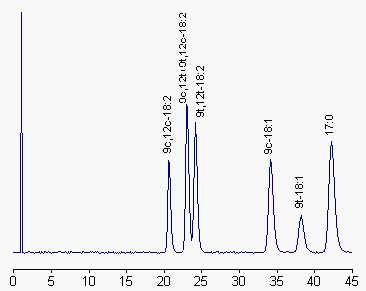
More efficient separations of cis and trans geometric and positional fatty acid isomers are obtained using silver ion HPLC, utilizing columns packed with bonded silica (phenylsulfonic acid groups) in which the sulfonic acid protons have been exchanged with Ag ions (Christie WW et al., J Chromatogr 1989, 469, 261). Applications of this technology to the fractionation of cis and trans oleic and linoleic acid methyl esters were recently reported (Adlof RO et al., J Chromatogr 1998, 799, 329; Adlof RO et al., J Chromatogr A 2002, 953, 293).
A review describes many analytical methods developed for the determination of trans-C18 fatty isomers in milk fat. Infrared spectroscopy, gas chromatography, thin-layer chromatography, liquid chromatography and mass spectrometry are used for these determinations (Ledoux M et al., Eur J Lipid Sci Technol 2007, 109, 891). Several aspects of the analysis of trans fatty acids with particular emphasis on CLA may be found in a review by Fritsche J et al. (Fett 1998, 6, 190) and by Juaneda P et al. (Eur J Lipid Sci Technol 2007, 109, 901).
A practical and precise guide with selected references should be consulted before running analyses of conjugated linoleic acid (Christie WW et al., Inform 2001, 12, 147-152). Similarly, the review by McDonald RE et al. (in Food Lipids, Akoh CC et Min DB Eds, M. Dekker, chap. 6, p.137, 1998) should be consulted before analyzing trans fatty acids. Improved separations of conjugated fatty acid methyl esters were described using silver ion HPLC (Sehat N et al., Lipids 1998, 33, 963; Sehat N et al., Lipids 1999, 34, 407). To help the identification of all isomers of cis/trans conjugated linoleic acid methyl esters from the 6,8- to 13,15-positions, their relative retention order has been determined using silver ion HPLC with two elution systems (Delmonte P et al., Lipids 2005, 40, 509).
As the choice of methylation reagent is critical in the analysis of conjugated fatty acids as methyl esters, specific derivatization methods involving sodium methoxide in methanol should be used (Shanta NC et al., J AOAC int 1993, 76, 644). Following extensive studies, it was shown that for the quantitative analysis of conjugated linoleic acid in lipid samples by GLC, proper methylation methods should be chosen on the basis of the chemical forms of these compounds in the samples (Park SJ et al., J Agric Food Chem 2002, 50, 989).
Synthesis and gas chromatography analysis of all the 6,8- to 13,15-cis/trans conjugated linoleic acid isomers (identified in food and dietary supplements) were also reported (Delmonte P et al., Lipids 2004, 39, 185). An optimum separation of 18:1 isomers were obtained with a CP-sil 88 column (Alves SP et al., J Chromatogr A 2009, 1216, 5130).
Gas-liquid chromatography combined with argentation thin-layer chromatography was shown to be very efficient in analyzing individual isomeric 18:1 acids in milk fats (Wolff RL et al., JAOCS 1995, 72, 1197; Precht D et al. Lipids 2001, 36, 827). The same technical procedure was shown to be successfully applied to dairy fats directly derivatized in the dry products (Golay PA et al., Food Chem 2006, 101, 1115). The limitation of that combined procedure has been described (Kiran CR et al., Grasas Aceites 2013, 64, 95).
The trans isomers of linoleic, linolenic and g-linolenic acids present in milk fat were shown to be efficiently separated using a 60 m Supelcowax column as well as short-chain saturated and monounsaturated fatty acid analogs (Kramer JKG et al., Lipids 2002, 37, 823). A quantitative determination of cis-9, trans-11 and trans-10, cis-12 isomers of the conjugated linoleic acid by gas chromatography using a 100 m capillary column has been reported (Zabala A et al., J Chromatography B 2007, 855, 152).
A rapid method for the determination of trans isomers of 16:1, 18:1, 18:2, 18:3, and 20:1 was described using GLC with a 30 or a 60 m capillary column (Shirasawa S et al., J Oleo Sci 2007, 56, 53). The determination of conjugated linoleic acid (cis-9,trans-11) in human plasma was accurately obtained using fast gas chromatography after convenient derivatization (Bondia-Pons I et al., J Chromatogr A 2007, 1157, 422). An efficient method for measuring c9,t11- and t10,c12-conjugated linoleic acid in milk was developed using a fast column (40 m) (Molto-Puigmarti C et al., Anal Chim Acta 2007, 602, 122).
The separation of trans isomers of eicosapentaenoic and docosahexaenoic acids using gas chromatography with a polyethylene glycol stationary phase has been reported (Mjos S, Eur J Lipid Sci Technol 2008, 110, 547).
The separation of conjugated trienoic acids (a-eleostearic, b-eleostearic and punicic acids) were isolated from total methyl esters by TLC using hexane/diethyl ether as the mobile phase (Devi PS, JAOCS 2003, 80, 315).
Separation and identification of 20-carbon metabolites of conjugated linoleic acid isomers with three or four double bonds were reported to be successful when reversed-phase HPLC is combined with silver-ion HPLC (Juaneda P et al., J Chromatogr 1999, 724, 213). A practical guide to the isolation, analysis and identification of conjugated linoleic acid may be consulted (Christie WW et al., Lipids 2007, 42, 1073).
Silver-ion HPLC was used in semi-preparative mode to separate two cis and trans geometric isomers of linoleic acid with a chemical purity higher than 96% (Adlof RO, J Chromatogr A 2004, 1033, 369-371).
An overview of methods for the determination of trans fatty acids using various technologies may be consulted (Ratnayake WMN, J AOAC Int 2004, 87, 523). A comparison of total trans fatty acid amounts in food obtained with different technologies (GC, infra-red analyses) has been made and shown that Fourier transform near-infrared spectroscopy has several advantages but requires comparisons with accurate GC determinations (Azizian H et al., Lipid Technol 2004, 16, 229). The advantages and disadvantages of the most popular derivatization methods has been concisely reviewed (Aldai N et al., J Sci Food Agric 2005, 85, 1073). Based on experiences, the authors put forward the trimethylsilyl-diazomethane method as an efficient procedure for ruminant tissue lipid determination. A clear review on the fundamentals, practical aspects and applications of infra-red analyses has been released by Pasquini C.
Preparation of trans fatty acids
An isomerization procedure has been described which allows the preparation of MUFA mixtures that can be used as reference material for GC analysis (Delmonte P et al., J Chromatogr A 2008, 1214, 30). Isomerized 17:1 can first be fractionated into cis and trans geometric isomers by Ag+-HPLC, and then each fraction can be separated into positional isomers by reversed-phase HPLC.
Preparation of Conjugated linoleic acid (CLA)
The CLA (t9, t11-18:2, c9, t11-18:2 and t9, t11-18:2) can chemically be obtained as standards from alkali-isomerized linoleate (Scholfield CR et al., JAOCS 1970, 47, 303). A mixture containing 72% c9,t11-18:2 and 26% c9,c11-18:2 was readily obtained through KOH-catalyzed dehydration of ricinoleic acid at 80°C with a 77% conversion efficiency (Yang L et al. Chem Phys Lipids 2002, 119, 23). The t10,c12-isomers may be prepared from the previous mixture by low-temperature crystallization in conjunction with urea treatment (Kim SJ et al., J Food Sci Nutr 2000, 5, 86). A preparation of trans,trans-isomers of linoleic acid may also be prepared by methylation with BF3/methanol in controlled conditions (Kim SJ et al., J Agric Food Chem 2003, 51, 3208). A stereoselective procedure for the synthesis of c10,12c-18:2, t10,t12-18:2 an t7,c9-18:2 has been described (Kellersmann C et al., Lipids 2008, 41, 777).
A simple method to prepare the most important isomers of linoleic acid (cis-9, trans-11 and trans-10,cis-12 isomers) was described by Ip et al (Cancer Res 1991, 51, 6118).
The procedure is as follows: 1 g of linoleic acid is added to a solution of 0.3 g NaOH in 58 g ethylene glycol. The mixture is then heated for 2 h under nitrogen at 180°C. After cooling and neutralization with HCl the fatty acids are extracted twice with hexane. After evaporation, a mixture containing about 50% of each isomer with a yield of about 92% is obtained.
A separation of the two conjugated isomers may be obtained using the ability of lipases produced by the fungus Geotrichum to selectively hydrolyze the cis-9,trans-11-18:2 methyl ester (Haas MJ et al., Lipids 1999, 34, 979).
A general cis-trans isomerization of polyunsaturated fatty acids may be obtained using p-toluenesulfinic acid has been described (Snyder JM et al., JAOCS 1982, 59, 469).
It must be noticed that infrared methodologies was developed for the rapid quantification of the total trans fatty acid levels in oils and fats (overview in Mossoba MM et al., J AOAC int 2004, 87, 540; Anal Bioanal Chem 2007, 389, 87; Delmonte P et al., Anal Bioanal Chem 2007, 389, 77). Using a novel Fourier transform infrared spectroscopic method, the lower limit of trans quantification was shown to be about 5% of total fat.
DICARBOXYLIC FATTY ACIDS
One of the simplest methods used to analyze diacids is to extract the tissues or fluids with acidified organic mixtures followed by a derivatization of the acids into phenacyl esters easily detected with UV, as for the simple fatty acids. Other procedures are reported, the most efficient but also the most sophisticated used methylated extracts analyzed by gas chromatography coupled with mass spectrometry (Passi s et al., Biochim Biophys Acta 1993, 1168, 190).
Procedure:
Free diacids are extracted using the classical Folch’s procedure but after the addition of 0.1 volume of 0.1 N H2SO4. The chloroform extract (lower phase) is dried by elution through a Pasteur pipette containing anhydrous sodium sulfate. After addition of a drop of triethylamine (about 1.5 equivalents), the solvent is evaporated and the residue is converted to phenacyl esters as explained before. Commercially obtained diacids are converted in parallel.
The esters solution is evaporated under nitrogen, the residue dissolved in 1 ml methanol, followed by 1 ml water. The esters are extracted by partition with a mixture of pentane/diethyl ether (1/1, v/v).
It is recommended to purify the extracts by TLC on silicagel plates run in hexane/diethyl ether/acetic acid (10/10/0.5, v/v). After a primuline spray, the spots corresponding to the diacid esters (Rf = about 0.3) are extracted 2 times with hexane/ether (1/1, v/v). The dried residue is dissolved in a little volume of acetonitrile/water (1/1, v/v) before HPLC analysis.
The samples are chromatographed on a 25 cm x 0.4 mm C18 column. The elution begins with acetonitrile/water (50/50, v/v) during 10 min, followed by a 40 min gradient from acetonitrile/water (1/1) to pure acetonitrile (may be followed by 10 min 100%ACN if necessary) at 1 ml/min. UV detection is set at 242 nm.
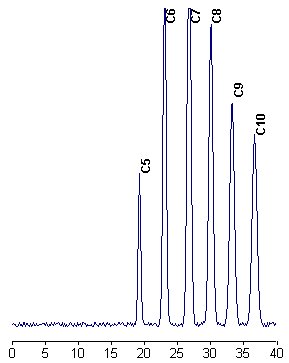
CYCLIC FATTY ACIDS
The HPLC procedures for the analysis of cyclic fatty acids (cyclopentenyl, cyclopropane, cyclopropene) were extensively reviewed by Dobson G (Lipid analysis in oils and fats, Hamilton RJ Ed, Blackie Acad and Professional, London, 1998, p.136).
Cyclopropenoid fatty acids are labile compounds. They are easily destroyed by heating and acid media during analysis and esterification process. Cold base-catalyzed esterification (30 seconds in methanolic KOH solution) followed by gas chromatography was shown to give good values of repeatability and recovery with no artifacts formation (Aued-Pimentel S et al., J Chromatogr A 2004, 1054, 235).
A quantitative method for the determination of cyclopropenoid fatty acids (malvalic, sterculic and dihydrosterculic acids) in cottonseed oil by HPLC was proposed (Obert JC et al., J Agric Food Chem 2007, 55, 2062). This reliable method involves extraction, saponification, and derivatization of the free fatty acids with 2-bromoacetophenone to give phenacyl esters.
The determination of furan fatty acids in plant oils may be done after transmethylation by normal phase HPLC coupled with capillary gas chromatography (Boselli E et al., J Agric Food Chem 2000, 48, 2868). A selective detection of furan fatty acids may be achieved by using a photoionization detector.
The stability of the cyclopropane ring and the fatty acid composition were studied using bacteria and fungi (Eras J et al., J Agric Food Chem 2008, 56, 4923). It was demonstrated that chlorotrimethylsilane and pentanol for 1 h at 80°C may be used to extract and derivatized normal and cyclic fatty acids in a single step with a good recovery (about 96%).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire