
These complex compounds (LPS) are the endotoxic O-antigens found in the cell walls of Gram-negative bacteria (S-lipopolysaccharides) but are also present in one fungus, they may cause several pathophysiological symptoms, such as fever, diarrhea, blood pressure decrease, septic shock, and death.
A lipid part (Lipid A) forms a complex with a core polysaccharide part through a glycosidic linkage. The core part is linked to a third external region of a highly immunogenic and variable O-chain polysaccharide or O-antigen made up of repeating oligosaccharide units. The latter region of the LPS molecule is responsible for bacterial serological strain specificity.
Lipid A consists of a backbone of b-1,6-glucosaminyl-glucosamine with two phosphoester groups in the 1-position of glucosamine I and in the 4-position of glucosamine II. The 3-position of glucosamine II forms the acid-labile glycosidic linkage to the long-chain polysaccharide. The other groups are substituted (in Escherichia) with hydroxylated fatty acids as hydroxymyristate (two ester-linked and two amide-linked) and normal fatty acids (laurate). The fatty acid profiles of lipopolysaccharides differ markedly from those of the phospholipids from the same species.
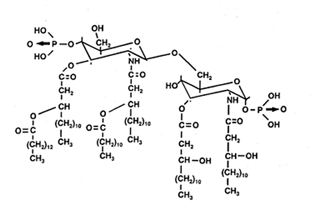
Structure of lipid A of Escherichia
Although the hydroxylated fatty acids are generally 3-hydroxyalkanoic acids, 2-hydroxyalkanoic acids are also common. Exceptionally, some bacteria seem to lack hydroxy acids such as some species of Gardnerella, Thermus, Thiobacillus, Bacteroides, Sorangium, Achromobacter, and Brucella. In most organisms, the major hydroxy acids are straight-chain, even-carbon compounds (from 12 to 18 carbon atoms). Odd-carbon acids are minor components (13 to 17 carbon atoms) in some enterobacteria, some Vibrio, some Pseudomonads and Bordetella. They are also found in Pectinatus, Veillonella and Selenomonas. Branched-chain hydroxy fatty acids are also present in Pseudomonas, Moraxella, Desulfovibrio, Capnocytophaga, Flavobacterium, Legionella species (3-OH i-11:0 up to3-OH i-17:0). Few unsaturated hydroxy fatty acids have been described, but 2-OH-18:1 is present in some Pseudomonas and Azospirillum species.
Studies have shown that the lipid A structure of some species (Francisella novicida), differs depending on growth temperature in in vitro cultures [12, 13]. Indeed, these bacteria synthesize a lipid A with longer fatty acids when grown at 37°C, the temperature of warm-blooded hosts, as compared to 18°C, a lower temperature encountered in arthropods or water environments (Shaffer SA et al., J Am Soc Mass Spectrom, 2007, 18, 1080).Other structure remodeling was observed in response to acidic pH stress. These changes were characterized by an increase in the relative abundance of molecular species bearing an additional hexose unit on the diglucosamine backbone, similar to species present when bacteria are grown under reduced environmental temperature (Robert CB et al., Biochimie 2017, 141, 16).
Lipopolysaccharides are the major toxins of Gram-negative bacteria (endotoxin). The only Gram-positive bacteria that possesses LPS is Listeria monocytogenes, an infective agent in milk. While the polysaccharide is non-toxic, the lipid A part is responsible for the toxic activities of these bacteria which result in septic shock.
For the first time, the existence of a lipopolysaccharide was demonstrated in a fungus, Antrodia camphorata, Polyporaceae (Cheng JJ et al., J Agric Food Chem 2005, 53, 469). Compositional analysis revealed that sorbitol, fucose, galactose, and glucose were the neutral sugars in that lipopolysaccharide and that galactosamine and glucosamine were absent. Furthermore, the isolated compound showed a low cytotoxicity and even some anti-inflammatory effect. These properties deserve further investigations of the reported therapeutic actions of the medicinal mushroom commercially available in Taiwan.
An efficient procedure for the extraction of LPS from bacteria and for the isolation of lipid A has been described by El Hamidi et al. (J Lipid Res 2005, 46, 1773). The method used hot ammonium isobutyrate solvent and was efficient in extracting pure lipid A ready for structural study.
A easy microextraction and a rapid method for mass spectrometric characterization of bacterial lipid A have been described (El Hamidi A et al., J Lipid Res 2005, 46, 1773).
An extensive review on lipopolysaccharides including their structure, function and bacterial identifications may be consulted for further details (Caroff M et al., OCL 2020, 27, 31).
One of the precursors of lipid A, lipid X, has been described and displayed some pharmacological properties.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire