
Ketones with various chain lengths are widely found in nature where they contribute to the flavors and odors in animals as well as in plants. Some short-chain ketones are responsible for mushroom odor, such as 3-octanone and 1-octen-3-one (Maga JA, J Agric Food Chem 1981, 29, 1). 3-Octanone is a volatile infochemical present in fungi and recognisable by fungivores (Holighaus G et al., Chemoecology 2014, 24, 57).It has been shown to have nematicidal role in the oyster mushroom (Pleurotus ostreatus). Octan-3-one was identified as the compound responsible for dispatching the nematodes in causing paralysis and cell death by disrupting cell membrane integrity (Lee CH et al., Sci Adv 2023, 9/3)
2-Decanone is concentrated in the oily extract of a Jamaican sponge, Plakortis sp. (Bowling JJ, et al., Chemoecology 2010, 20, 207). It strongly inhibited attachment of bacteria and mussels. This antifouling property may be used in improving this product into coating design.
2-Heptadecanone is found in the web of some spiders (Pholcidae) to attract females (Schulz S, J Chem Ecol 2013, 39, 1).
Two alkyl ketones were shown to be released as pheromones by adult female spiders of the family Agnelidae : 6-methyl-3-heptanone and 8-methyl-2-nonanone (Schulz S, J Chem Ecol 2013, 39, 1).
1-Hydroxyoctan-3-one was shown to exhibit a higher concentration in spoiled apple purees than in control ones and could thus be relevant markers of fruit spoilage. A Penicillium digitatum strain appeared to be the highest 1-hydroxyoctan-3-one producer (Mouriot H et al., Food Chem 2025, 146482),
The development of a fresh mushroom off-flavor in wines, a recurring problem stemming from fungal alteration on grapes, has been linked to the presence of 1-octen-3-one and its metabolite octane-1,3-diol (Ployon S et al., Food Chem 2025, 482, 143454). This provides a fast and reliable diagnostic tool for winemakers.
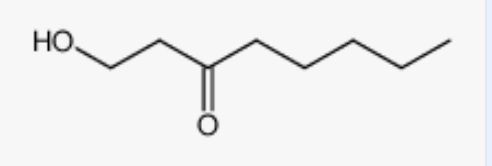 1-Hydroxyoctan-3-one
1-Hydroxyoctan-3-one
As examples, several 2-methyl ketones, with a 9 to 15 carbon-chain length, were identified in milk and milk products. Some of them are also present in brandies, where they are produced through b-oxidation and decarboxylation of fatty acids by yeast. One of 2-methyl ketones, 2-tridecanone, is secreted by the glandular trichomes in the tomato leaves and as an insecticide makes the plant resistant to a variety of insects.
A hydroxylated alkyl ketone, named CAI-1, 3-hydroxytridecan-4-one, was determined to be the main auto-inducer (quorum sensing substance) in Vibrio cholerae (Higgins DA et al., Nature 2007, 450, 883). At high cell density, the presence of that auto-inducer represses both the expression of virulence factors and the formation of biofilms. Thus, these findings suggest that this signaling molecule could be used as a therapy to prevent cholera infection.
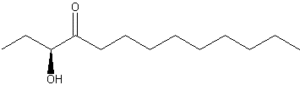
3-Hydroxytridecan-4-one (CAI-1)
3-Methyl-2,4-nonanedione is a volatile compound with an intense odor of anise, fruit pit, and dried parsley that was as an off-flavor in reversed soybean oil reminiscent of beany and green off-odor. The role of light in the formation of this compound was demostrated in soyabean oil. Its content is low in high in oxidized red wines, systematically exceeding the perception threshold (62 ng/L) and reaching a maximum of 340 ng/L Its sensory impact and the wide range of levels at which it occurs according to the beverage drew the attention of the scientific community (Pons A et al., Food Chem, online 18 February 2026, 148484).
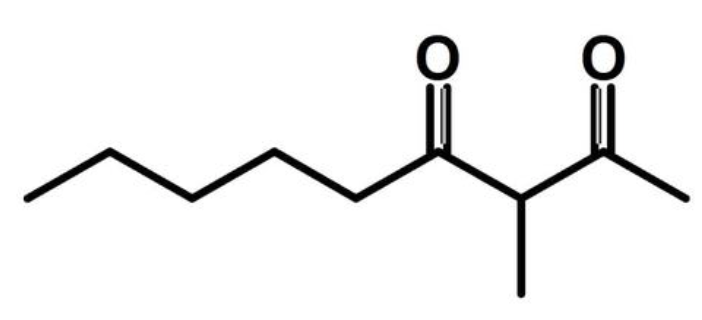
3-Methyl-2,4-nonanedione
During the isolation and evaluation of the antimicrobial activity of Streptomyces metabolites active against phytopathogens, one new diketone, streptoone A, and a new ketonic acid, streptoone B were isolated from Streptomyces sp. SN0280 (Tian H et al., J Nat Prod 2017, 80, 1015).
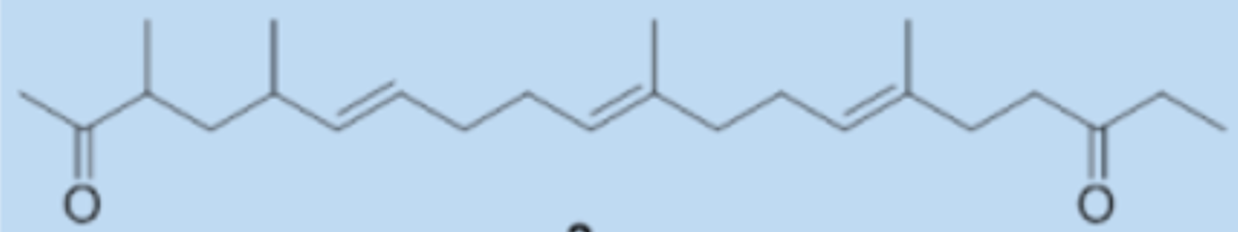
Streptoone A
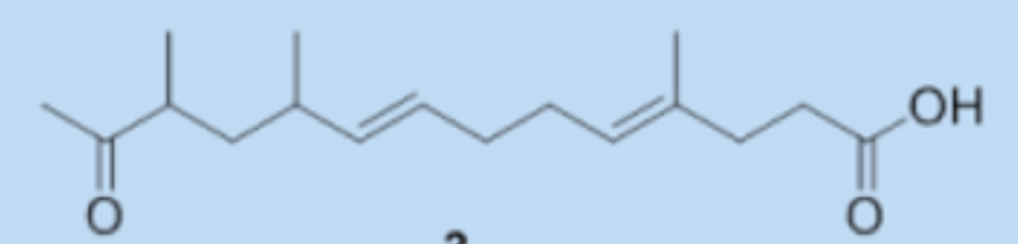
Streptoone B
Streptoone A displayed antibacterial activity (MIC value of 7.81 μg/mL) against Clavibater michiganensis, comparable with the positive control streptomycin while Streptoone B showed antifungal activity (MIC value of 15.63 μg/mL) against Phytophthora capsici (positive control carbendazol MIC value of 7.81 μg/mL). These molecules provide new templates for the potential treatment and management of these phytopathogens.
Several unsaturated ketones with one or two triple bonds (ketoalkenynes) have been identified in lipid extracts of Echinacea (Barnes J et al., J Pharm Pharmacol 2005, 57, 929). With some alkylamides, these compounds are at the basis of the use of these plants to treat infections, to aid wound healing and to enhance the immune system. Among these ketones, the most active has 15 carbon atoms one double and two triple bonds (pentadeca-8-ene-11,13-diyn-2-one) and was determined to be responsible for inhibitory effects on NO and PGE2 production by macrophages (Zhang X et al., Phytochemistry 2012, 74, 146). One of the acetylenic ketones extracted from E. pallida, pentadeca-8,13-dien-11-yn-2-one is the most cytotoxic when tested on human cancer cell lines (Chicca A. et al., Brit J Pharmacol 2008, 153, 879).
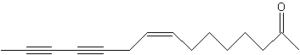
Pentadeca-8-ene-11,13-diyn-2-one
Other similar but less active compounds, some of them being hydroxylated, are present in lipid extracts of Echinacea.
They are commonly found in epicuticular wax at the surface of many angiosperms. They have the common formula :
[CH3(CH2)n]2C=O with n = 3-20
One of the most common of these symmetrical ketones is palmitone (with n = 14).
These compounds are known to have sex pheromone activities in mammals, such as civetone(1-cycloheptadecen-10-one) and muscone (1-methyl-cyclopentadecan-3-one). Muscone has demonstrated neuroprotective effects in various disease models, including cerebral ischemic stroke, Alzheimer’s disease, and diabetic peripheral neuropathy. Its role in Parkinson’s disease in inhibiting ferroptosis has been demonstrated (Xu M et al., Free Rad Biol Med 2025, 239, 298).
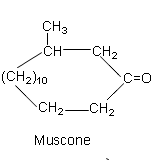
Furthermore, it was shown that the odor of cyclic ketones in related to the ring size.
2-Ethyl-5-propylcyclohexan-1,3-dione (chiloglottone1) is produced by orchids which employ sexual deceit to attract males of their pollinator species (Frank S et al., PNAS 2009, 106, 8877). This specific volatile signal mimics female-released sex pheromones and proved to be important in the relations between orchids of the genus Chiloglottis, native to Australia, and their pollinator species.
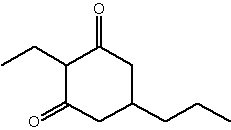
Chiloglottone1
In 1972, LeTellier et al. reported that a group of compounds known as 2-alkylcyclobutanones is formed from triacylglycerols on irradiation and are found in treated foods (LeTellier PR et al., Lipids 1972,7, 75). Indeed, it has been demonstrated that treatment of food products with a low dose of ionising radiation followed by suitable storage will reduce bacterial loads and increase shelf-life of the products. There is a debate on the health risks related to the consumption of irradiated foods containing.
These 2-alkylcyclobutanone compounds have the same number of carbon atoms as their precursor fatty acids and the cyclobutanones formed from palmitic, stearic, oleic and linoleic acids are 2-dodecyl-, 2-tetradecyl-, 2-tetradecenyl- and 2-tetra-decadienylcyclobutanone, respectively.
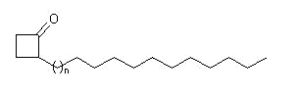 2-Alkylcyclobutanones
2-Alkylcyclobutanones
The evidence of the natural existence of 2-tetradecenylcyclobutanone and 2-tetradecylcyclobutanone in non-irradiated cashew nut and 2-decylcyclobutanone in nutmeg was demonstrated, thus disproving the hypothesis that these compounds are unique radiolytic products (Variyar PS et al., J Agric Food Chem 2008, 56, 11817).
A method for the detection of irradiation treatment published by the European Committee for Standardization, consists in the determination of 2-alkylcyclobutanones, but it may only be applied with complete confidence to foodstuffs treated for the purpose of microbial disinfection (doses >0.5 kGy), whose fat content is higher than 1.0 g % (Ndiaye B et al., Rad Phys Chem 1999, 55, 437).
Formation of long-chain ketones containing from 29 to 35 carbon atoms are formed by pyrolysis of free fatty acids or triacylglycerols.
Experimentation involving heating of oleic acid and palmitic or stearic acid at temperature higher than 300°C provided evidence of the formation of mixtures of ketones with 33 or 35 carbon atoms (Evershed RP et al., Tetrahedron Lett 1995, 36, 8875).
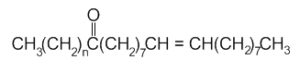
n = 14 or 16
These compounds were studied to determine the origin of organic residues preserved in archaeological pottery but they may originate from both animal tissues or higher plant leaf waxes.
These long-chain unsaturated methyl or ethyl ketones are very important compounds which are produced by a specific class of phytoplankton represented notably by the coccolithophorid Emiliana huxleyi (Volkman JK et al., Phytochemistry 1980, 19, 2619), the most abundant unicellular phytoplankton which plays a fundamental role in the total primary production in the oceans. Satellite images clearly demonstrate that point. The Emiliana blooms have a major impact on the biological carbon cycle in the ocean and on ocean/atmosphere fluxes of carbon dioxide (Westbroek et al., Global Planetary Change 1993, 8, 27).

Emiliana huxleyi
Alkenones possess several unusual characteristics, including their very long chain-length (C35-C40) and the spacing (C7) and configuration (trans) of their positions of unsaturation (Marlowe IT et al., Chem Geol 1990, 88, 349) and they were shown to be membrane-unbound lipids (Sawada K et al., Phytochemistry 2004, 65, 1299).
The two most abundant alkenones (C37:2 and C37:3) have so far unknown physiological function but their characteristic is that they remain partially intact in oceanic sediments after the cellular death and thus may be used as biomarkers.

Until now they were reported exclusively from the oceanic haptophytes Emiliana huxleyi and Gephyrocapsa oceanica and from coastal species of Chrysotila (Rontani JF et al., Phytochemistry 2004, 65, 117).
As for the phospholipid fatty acids in all living cells, the proportion of the more unsaturated alkenone is increased when the growth temperatures get colder and vice versa. Thus, a simple index ([37:2]/{[37:2]+[37:3]}) was formulated to quantify the degree of unsaturation in a given alkenone series and shown to be linear versus the growth temperature (Prahl FG et al., Nature 1987, 330, 367). Consequently, stratigraphic measurement of that index in dated sediments is done by paleoceanographers to assess climate changes on timescales ranging from interannual (El Nino) to millenial (glacial/interglacial). Thus, the variations in sea-surface of the eastern equatorial Atlantic over the past 500,000 years were inferred from the distribution of alkenones in sediments (Brassell SC et al., Nature 1986, 320, 129).
Similarly, the dissolved carbon dioxide concentration of surface waters in which E. huxleyi grew can be calculated using the proportion of the two different isotopic forms, 12C and 13C.
Thus, for their peculiarities, alkenones are not only the most abundant extractable lipids in Quaternary marine sediments but also the most precious molecules for the knowledge of past sea-surface temperature.
Methyl-branched alkenones with up to five double bonds were characterized in saline lake sediments (Liao S et al., Org Geochem 2021, 156, 104243). Further analyses revealed that the degree of unsaturation of these branched alkenones is also sensitive to temperature.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire