
The main component of this group is the diacyl derivative:
1,2-diacyl-sn-glycero-3-phosphorylcholine or phosphatidylcholine or lecithin.
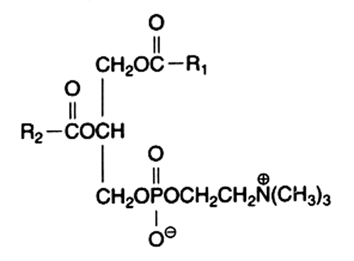
R1 and R2 are identical or different fatty acids
Phosphatidylcholine is abundant in all cell extracts, it frequently forms about half of all membrane phospholipids in animal preparations. In animal cells, the fatty acid from the 1-position is frequently 16:0 and that from the 2-position is 18:1 or 18:2, exceptionally more unsaturated fatty acids are found. Choline has a pK near 13.9 and produces a polar head group with a strong zwitterionic character over the entire pH range.
While the diacyl form is the most abundant, other forms can be found in cell extracts.
It is well known that dipalmitoyl phosphatidylcholine (DPPC) is the main lipid of the pulmonary surfactant, about 50% in weight (Agassandian M et al., Biochim Biophys Acta 2013, 1831, 612). Its function is dependent on its high gel to sol phase transition temperature (41°C) and consequently, DPPC is a solid material at body temperature. It is well known that it confers surface-tension lowering activities. The situation is not the same in the gastric mucus where palmitoyl-oleyl and palmitoyl-linoleyl phosphatidylcholines are the most abundant, DPPC amount being lower than 3% (Bernhard W et al., Biochim Biophys Acta 1995, 1255, 99).
It has been shown that a structured phosphatidylcholine, 1-acetyl,2-DHA-glycero-3-phosphorylcholine, is more efficient than free DHA in allowing this fatty acid crossing blood-brain barrier and in preventing brain lesions after an ischemic stroke (Lagarde M et al., OCL 2016, 23, D102).
After the first observations of diacylglycerol formation by hormone-stimulated hepatocytes (Hughes BP et al., Biochem J 1984, 222, 535; Bocckino SB et al., J Biol Chem 1985, 260, 14201), it became progressively evident that phosphatidylcholine plays an important and general role in cell signalling. The first unambiguous demonstration was that of a rapid formation of diacylglycerol from phosphatidylcholine in stimulated pre-adipocytic 3T3 cell line (Besterman JM et al., PNAS 1986, 83, 6785). Various signal transduction systems have been investigated and have demonstrated that in endothelin-1 stimulated vessels (Liu GL et al., J Vasc Res 1999, 36, 35), in PDGF-induced activation of MAPK pathway in fibroblasts (Van Dijk MC et al., J Biol Chem 1997, 272, 11011), in thyroxine stimulation of liver cells (Kavok NS et al., BMC cell Biol 2001, 2:5) and in prostaglandin-induced proliferation of osteoblasts (Sakai T et al., J Bone Miner Metab 2004, 22, 198), a production of diacylglycerol species from phosphatidylcholine is generated.
A novel family of oxidized phosphatidylcholine was shown to serve as ligands for the macrophage scavenger receptor CD36 (Podrez EA et al., J Biol Chem 2002, 277, 38503). These structures were shown to derived from phosphatidylcholine molecules having C16:0 at the sn-1 position and either C18:2n-6, C20:4n-6 or C22:6n-3 at the sn-2 position. Thus, the species active on CD36 have been identified to have an sn-2 acyl group that incorporates a terminal g-hydroxy (or oxo)-a,b-unsaturated carbonyl (alcohol or aldehyde group). As an example, the species shown below is generated from oxidation of a docosahexaenoic acid ester. The compounds generated from different precursors are similar except for the chain length of the truncated oxidized fatty acid at the sn-2 position.
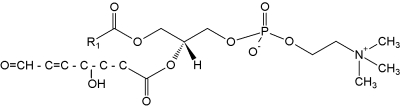
These truncated phosphatidylcholine species are likely involved in the CD36-mediated recognition of oxidized lipoproteins and foam cell formation in vivo. Thus, this novel family of oxidized phospholipids serve as ligands for the macrophage scavenger receptor CD36 (Podrez EA et al., J Biol Chem 2002, 277, 38503).
It has been suggested that they may be also potential targets for antimicrobial peptides (Mattila JP et al., Biochim Biophys Acta 2008, 1778, 2041). Afterwards, a protrusion of oxidized fatty acids from the hydrophobic membrane interior into the more polar aqueous compartment was demonstrated, thus facilitating physical contact between pattern recognition receptor and molecular pattern ligand (Li XM et al., Biochemistry 2007, 46, 5009). These observations led to the proposition of a new model of the structure of oxidized cell membranes, the lipid whisker model (Greenberg ME et al., J Biol Chem 2006, 283, 2385).
Oxidized phospholipids are the result of a series of radical catalyzed chemical reactions and display physiopathological roles in disease development as in age-related and chronic diseases, atherosclerosis, inflammation, immune responses and various neurodegenerative diseases. Evidence is progressively growing that oxidized phosphatidylcholines are potent agonist for peroxisome proliferator-activated receptor gamma (review in : Konger RL et al., Prost Lipid Med 2008, 87, 1). These compounds, generated in the photoreceptor outer segment by photo-oxidation, may serve as a physiological signal for CD36-mediated phagocytosis, thus enabling retinal remodeling (Sun M et al., J Biol Chem 2006, 281, 4222). Immunological detection of high levels of carboxyethylpyrrole modifications of proteins from the retinas of individuals with age-related macular degeneration provided presumptive evidence for the involvement of docosahexaenoate-derived oxidatively truncated phospholipids in retinal pathology. Free-radical oxidation of human plasma low-density lipoprotein (LDL) produces (carboxyalkyl) pyrrole (CAP) epitopes that were detected with enzyme-linked immunosorbent assays. Oxidation of linoleic acid-PC formed human serum albumin-bound 2-(w-carboxyheptyl)-pyrrole, while oxidation of arachidonic acid generated 2-(w-carboxypropyl)pyrrole (Kaur K et al., Chem Res Toxicol 1997, 10, 1387).
Oxidized phosphatidylcholine can initiate and modulate many cellular events linked to inflammation and atherosclerosis. As they may be accumulated in vivo, suggestions have been made that they could be biomarkers reflecting these diseases (Ashraf MZ et al., Int J Biochem Cell Biol 2009, 41, 1241). A spectrometry technique has been developed for the determination of several species of oxidized phosphatidylcholine generated in a myocardium ischemia-reperfusion model (Nakanishi H et al., J Chromatogr B 2009, 877, 1366). The synthesis of truncated docosahexaenoate phpospholipids, their generation and peptide adduction chemistry have been desscribed (Gu X et al., J Org Chem 2003, 68, 3749).
Chronic alcohol exposure has been shown to increase circulating bioactive oxidized phosphatidylcholines (Yang L et al., J Biol Chem 2010, 285, 22211). These pro-inflammatory truncated phospholipids are correlated with steatohepatitis and tumor necrosis factor-alpha deposition in liver.
A review of the nature, biological activities and analysis of phosphatidylcholine and other phospholipids may be consulted (Domingues MR et al., Chem Phys Lipids 2008, 156, 1).
It was shown that a phosphatidylcholine species with esterified sn-2-azelaidic acid was the most common (2/3rd) phospholipid oxidation product of oxidized low density lipoprotein (Tokumura A et al., Lipids 1996, 31, 1251). Azelaic acid, a 9-carbon dicarboxylic acid, is formed by oxidative fragmentation of the 9,10-double bond of the most abundant unsaturated (oleoyl) and polyunsaturated (linoleoyl and linolenoyl) fatty acyl residues of LDL (Frankel EN, Prog. Lipid Res 1984, 23, 197). It is noteworthy that oxidized LDL levels in carotid plaque are 70-fold greater than circulating levels (Nishi K et al., Artherioscler Thromb Vasc Biol 2002, 22, 1649). It was further demonstrated that these phospholipid oxidation products target intracellular mitochondria to activate the intrinsic apoptotic cascade (Chen R et al., J Biol Chem 2007, 282, 24842). Later, these compounds depolarized mitochondria and allowed NADH loss, but did not inerfere with complex I function (Chen R et al., J Biol Chem 2009, 284, 26297).
It was later shown that molecular species possessing a fatty acyl hydroxyalkenal group can undergo a slow transformation into a novel oxidized species with a sn-2 acyl group incorporating a terminal furan moiety (Gao S et al., J Biol Chem 2006, 281, 31298). One of these species is shown below.
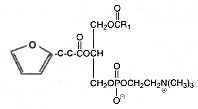
These derivatives were shown to be generated at sites of enhanced oxidant stress, such as within brain tissues. This was the first reported description of furan-containing phospholipids as endogenous products in a mammalian system. In contrast to their hydroxyalkenal precursors, these furan derivatives lack CD36 binding activity.
The alk-1-enyl-acyl derivatives (1-alk-1′-enyl-, 2-acyl-sn-glycero-3-phosphorylcholine) are also named choline plasmalogen (or plasmenylcholine). As the first carbon of glycerol is linked to the carbon chain through a -C-O-C=C- vinyl ether bond, these lipids are known as ether-linked lipids or ether lipids. The vinyl ether bond is acid labile. This form is present in appreciable quantity in some tissues as heart muscle (up to 40% of choline glycerophospholipids), in seminal fluid, and in smaller amounts in nervous system, platelets or red blood cells. The presence of this type of lipid was detected around 1930 by Feulgen R in histological sections where a positive reaction with the fuchsine-sulfurous acid reagent was observed in the cellular cytoplasm. The detected aldehyde was formed from the broken vinyl ether bond, the rest of the molecule giving a lyso-phospholipid (1-lyso 2-acyl phospholipid). This reaction was mainly due to the more abundant ethanolamine plasmalogen (or plasmenylethanolamine). Choline plasmalogen was the first aldehydogenic lipid to be isolated in a pure form (Gottfried et al., Fed Proc , 1961, 20, 278).
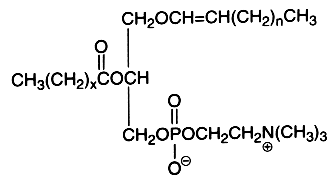
The first carbon chain is of the vinyl ether group, n being usually equal to 13-15. The second carbon chain is an esterified fatty acid with x being equal to 14-16 and possibly with one or two double bonds.
In plasmalogens from sheep heart, it was shown that the alkenyl chains are mainly composed of 16:0, 18:0, and 18:1n-3 but also of several trans isomers of 18:1. The t11-18:1 (vaccenic acid) was the most abundant (about 5%) but several others have their ethylenic bond spanning from C6-C8 to C16, which were similar to those isolated from sheep adipose tissue. Furthermore, several trans-16:1 alkenyl chains could be observed (ca. 1%), cis-16:1 isomers being present in trace amounts (Wolff RL, Lipids 2002, 37, 811)
The vinyl ether bond is very sensitive to acid treatment which generates a free long-chain aldehyde. When the acid treatment is made in the presence of methanol, it generates dimethyl acetals.
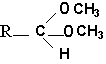
These derivatives are stable, they can be purified and analyzed by gas liquid chromatography.
The physiological function of these compounds remains poorly known. It was reported that thrombin treatment of endothelial cells activates a selective hydrolysis (phospholipase A2) of choline plasmalogen containing arachidonic acid in the sn2 position (85% of this phospholipid contain arachidonate) , thus releasing a lysoplasmenylcholine and a fatty acid leading to eicosanoid production (Creer MH et al., Am J Physiol 1998, 275, C1498). Similar observations were made in thrombin-stimulated platelets (Beckett CS et al., Thromb Res 2007, 120, 259). Simultaneously, it has been shown that lysoplasmenylcholine accumulated in hypoxic myocytes, producing electrophysiological alterations which would likely contribute to the initiation of arrhythmia (McHowat J et al., Am J Physiol Cell Physiol 1998,274, C1727). It was later shown that this specific cellular action results from an alteration in calcium cycling (Liu SJ et al., Am J Physiol Cell Physiol 2003, 284, C838). In opposition, a calcium-independent plasmenylcholine hydrolysis was described in hypoxic human coronary artery endothelial cells (Meyer MC et al., Am J Physiol Cell Physiol 2007, 292, C251). The vinyl-ether bond of plasmalogens is susceptible to attack by myeloperoxidase-produced HOCl and yields a lysophospholipid and a bioactive a-chloro-fatty aldehyde.
A decrease in the biosynthesis of plasmalogens is observed in several peroxisome biogenesis disorders such as the Zellweger syndrome, the neonatal adrenoleukodystrophy and some form of chondrodysplasia (Steinberg SJ et al., Biochim Biophys Acta 2006, 1763, 1733).
The role of choline plasmalogens in neurological diseases and in metabolic and inflammatory disorders has been reviewed (Wallner S et al., Chem Phys Lipids 2011, 164, 573).
The characterization of serum ether glycerophospholipids verified the specificity of choline plasmalogens, particularly the ones with 18:1 in sn -2, as a sensitive biomarker for the atherogenic state (Nishimukai M et al., J Lipid Res 2014, 55, 956). It has been demonstrated that serum choline plasmalogen with 18:1 or 18:2 in sn-2 among all choline plasmalogen molecular species showed the strongest correlations with a wide range of atherogenic parameters in human subjects (Nishimukai N. et al., Clinica Chimica Acta 2014,437, 147).
The alkyl, acyl derivative (1-alkyl-,2-acyl-sn-glycero-3-phosphorylcholine) is another ether lipid (saturated ether type) present in all cells but in low amounts (in general higher than those of choline plasmalogen). Appreciable quantities (20-50%) occur amongst the choline phospholipids of Tetrahymena, some mollusks, crustacea and various vertebrate tissues. The ether bond in these lipids is acid and alkali stable, the complete hydrolysis of the molecule giving 2 types of glycerol ether: 1-O-hexadecyl-sn-glycerol (chimyl alcohol) and 1-O-octadecyl-sn-glycerol (batyl alcohol).
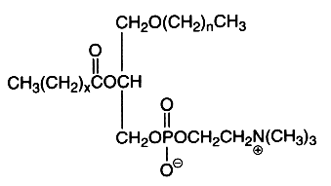
The first carbon chain is of the saturated ether form where n represents 15 or 17, while x is usually 14 or 16 with one or two double bonds.
An important derivative of this ether lipid is the Platelet Activating Factor (PAF) which is a 1-alkyl-,-2-acetyl-sn-glycero-3-phosphocholine, lipid mediator playing through a specific receptor a role in inflammatory and immune responses, and in platelet aggregation. It was also shown to be present in spermatozoid membrane and to be able to increase their mobility.
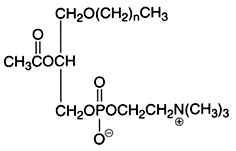
This is the structural formula of PAF where n is 15, or 17, in this case with most frequently one double bond.
In addition to enzymatically catalyzed PAF production, PAF receptor ligands are also generated non-enzymatically, and thus not subject to cellular control. Several derivatives, including PAF itself, may be generated by oxidative reactions. It has been shown that PAF and analogues are biologically active mediators for ultraviolet radiation-mediated effects in skin (review in : Konger RL et al., Prost Lipid Med 2008, 87, 1).
A glycosylated derivative of PAF has been designed in introducing an inositol molecule in the sn-2 position instead of an acetyl group (Danker K et al., Brit J Pharmacol 2010, 160, 36). This compound has an efficient anti-proliferative effect without cytotoxicity. This could be a promising drug for the treatment of hyperproliferative and migration-based skin diseases.
Furthermore, ether glycerophospholipids may have one or two hydrocarbon chains linked to glycerol by an ether bond (Paltauf F, Chem Phys Lipids 1994, 74, 101).
Ether lipids have been shown to have anti-tumor properties (apoptotic activity), including reduction of tumor cell invasion and inhibition of tumor metastases. Several analogues were synthesized in order to have very low rates of metabolism. Below are shown two very active molecules (edelfosine and ilmofosine) analogous to PAF but lacking the hydrolyzable ester functionality at the sn-2 position.
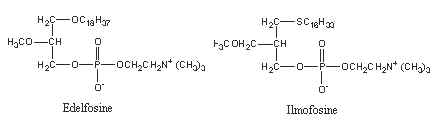
A number of other analogues were synthesized as they display antitumor activity after being taken up specifically by malignant cells. Fluorescent analogues were also synthesized as they are very useful for unveiling their mechanism of action and therapeutic targets (Quesada E et al., J Med Chem 2004, 47, 5333). The mechanism of inhibition of cell signaling pathways by these antitumor ether lipids has been reviewed in 1998 (Arthur G et al., Biochim Biophys Acta 1998, 1390, 85) and in 2013 (Van Blitterswijk W et al., Biochim Biophys Acta 2013, 1831, 663).
Monoacyl derivatives (lysoglycerophosphorylcholine)
This is a minor component (1 to 5%) of the tissue phospholipids. 1-acyl or 2-acyl derivatives may arise because of the action of specific phospholipases. They are very polar and have detergent properties.
Here is shown the 2-lysophosphatidylcholine produced by phospholipase A2 hydrolysis. This action is intimately associated with the signal transduction pathway in all types of cells (an unsaturated fatty acid is liberated and may be metabolized in various derivatives by other enzymes). A phospholipase A1 is able to cleave the sn-1 ester bond and generate a 1-lysocompound. These enzymes are used to study the phospholipid structure.
Lysophosphatidylcholine was also shown to be a selective chemoattractant for mononuclear leukocytes (Quinn MT et al., Proc Natl Acad Sci USA 1988, 85, 2805) and to be a pathological component of oxidized LDL in plasma (Parthasarathy S et al., Atheriosclerosis 1989, 9, 398) and of atherosclerotic lesions (Portman OW et al., J Lipid Res 1969, 10, 158).
More recently, it was reported that lysophosphatidylcholine was able to promote mature dendritic cell generation through G protein-coupled receptors on differentiating monocytes (Coutant F et al., J Immunol 2002, 169, 1688). This new cell signaling process opens perspectives for the understanding and treatment of acute and chronic inflammatory diseases. In this context, lysophosphatidylcholine containing DHA at the sn-1 position has been shown to be anti-inflammatory in relation with the reduced formation of leucotriene (LTC4) and cytokines (TNF-a and IL-6) (Huang LS et al., Lipids 2010, 45, 225). The suppressive effect was greater when the docosahexaenoic acid was hydroxylated on the carbon 17 (Jin MC et al., Eur J Lipid Sci Technol 2012, 114, 114). DHA from lysophosphatidylcholine was the most highly taken up (10-fold) by rat brain compared with its non-esterified form, a preference that was not found for the uptake of DHA by other organs such as the heart, kidney and liver (Thiès F et al., Am J Physiol 1994, 267, R1273)
Human NKT cells were shown to recognize lyso-phosphatidylcholine (and lyso-sphingomyelin) as antigens presented by CD1d molecules (Fox LM et al., Plos Biology October 2009).
It has been shown that spermatozoa from obese men are particularly rich in lysophosphatidylcholine (Pyttel S et al., Chem Phys Lipids 2012, 165, 861).
Lysophosphatidylcholine was demonstrated to be one product from the initial attack of plasmenylcholine by HOCl produced by the phagocytic enzyme myeloperoxidase, another one is a chlorinated fatty aldehyde (Albert CJ et al., J Biol Chem 2001, 26, 23733). Then, it was shown that the double bonds present in the fatty acid in the sn-2 position may be the secondary targets of HOCl leading to the production of lysophosphatidylcholinechlorohydrins (Messner MC et al., Chem Phys Lipids 2006, 144, 34).
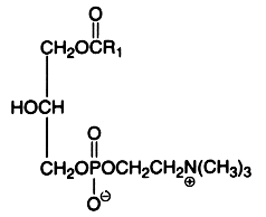
1-lyso-2-arachidonoyl-glycerophosphocholine bis-chlorohydrin
The attack of the vinyl ether bond of lysoplasmenylcholine by reactive chlorinating species results also in the production of secondary chlorinated fatty aldehydes, mainly 2-chlorohexadecanal (Albert CJ et al., J Biol Chem 2001, 26, 23733). According to the fatty acid structure, various chlorinated fatty acids are formed.
Sulfonium analog of phosphatidylcholine
A phosphatidylcholine analog containing a sulfur atom replacing the nitrogen atom of choline has been described in marine diatoms and algae (Bisseret P et al., Biochim Biophys Acta 1984, 796, 320). Thus, only two methyl groups are present at the end of the polar head : – S+(CH3)2 instead of – N+(CH3)3 . Furthermore, this phosphatidylsulfocholine, discovered in the diatom Nitzschia alba, completely replaces phosphatidylcholine in this species (Anderson R et al., Biochim Biophys Acta 1978, 528, 77). Methionine was shown to supply the S atom as well as both methyl groups of the dimethyl sulfonium moiety of the molecule.
Phosphatidyl alcohols are anionic lipids, which can be formed by the action of phospholipase D on mainly phosphatidylcholine in the presence of primary alcohols (mostly ethanol).
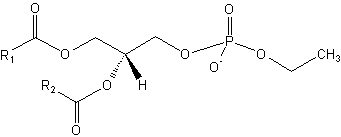
Phosphatidyl ethanol
Significant amounts of phosphatidyl ethanol have been shown to be formed in rat after ethanol exposure (Alling C et al., Biochim Biophys Acta 1984, 793, 119) and are also formed in chronic alcoholics (Hansson P et al., Alcohol Clin Exp Res 1997, 21, 108). Phosphatidyl ethanol has been shown to accumulate in lipoproteins (Liisanantti MK et al., Atherosclerosis 2005, 180, 263). It has been postulated that phosphatidyl ethanol-enriched HDL even could influence the progression of atherogenesis (Liisanantti MK et al., Alcohol Clin Exp Res 2009, 33, 283). Phosphatidyl alcohols are normally physiologically scarce, but when formed, they can affect physical properties of biological membranes (Jaikishan S et al., Biochim Biophys Acta 2010, 1798, 1615).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire