
MONOENOIC FATTY ACIDS
In his classic studies on fatty acids from pork fat, Chevreul (1823) recognized the nature of oleic acid but it was not prepared in pure condition for a long time. Its structure was not definitively elucidated by a series of complex chemical reactions until later (Baruch J, Ber 1894, 27, 172). Much simpler proof of the now accepted structure of oleic acid was given by means of oxidation techniques (Edmed FG, J Chem Soc 1898, 73, 627). The ozonisation method for the determination of the position of the unsaturated linkage was used for the first time in lipidology in 1903 (Molinari E, Annuario della Soc Chimica di Milano 1903, 9, 507). Oleic acid synthesis was realized in 1934 (Noller CR et al., J Am Chem Soc 1934, 56, 1563).
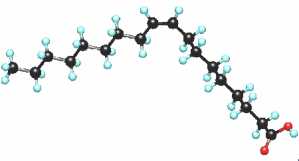
Mono-unsaturated normal fatty acids are widespread in the living world where they occur mostly as the cis-isomer. Over a hundred naturally occurring monoene fatty acids have been identified. They have the general structure:
CH3(CH2)xCH=CH(CH2)yCOOH
The most frequently they have an even number of carbon atoms and the unique double bond may be in a number of different positions.
The double bond can exist in two stereoisomeric forms :
The commonest cis-monoenes are of the n-9 series, as oleic acid from olive oil (cis-9-octadecenoic acid) and from quite all seed oils.
Some important monoenoic acids are found below:
|
Systematic name |
Trivial name |
Shorthand designation |
Molecular wt. |
Melting point (°C) |
| cis-4-decenoic | obtusilic | 10:1 (n-6) | 170.3 | |
| cis-9-decenoic | caproleic | 10:1 (n-1) | 170.3 | |
| cis-5-dodecenoic | 5-lauroleic (denticetic) | 12:1 (n-7) | 198.4 | |
| cis-4-dodecenoic | linderic | 12:1 (n-8) | 198.4 | |
| cis-9-tetradecenoic |
myristoleic |
14:1 (n-5) |
226.4 |
– |
| cis-5-tetradecenoic | physeteric | 14:1 (n-9) | 226.4 | |
| cis-4-tetradecenoic | tsuzuic | 14:1 (n-10) | 226.4 | |
| cis-9-hexadecenoic |
palmitoleic |
16:1 (n-7) |
254.4 |
0.5 |
| cis-6-hexadecenoic | sapienic | 16:1 (n-10) | 254.4 | |
| cis-6-octadecenoic |
petroselinic |
18:1 (n-12) |
282.4 |
30 |
| cis-9-octadecenoic |
oleic |
18:1 (n-9) |
282.4 |
16.2 |
| tr-9-octadecenoic | elaidic | tr18:1 (n-9) | 282.4 | 43.7 |
| cis-11-octadecenoic |
vaccenic (asclepic) |
18:1 (n-7) |
282.4 |
39 |
| cis-9-eicosenoic |
gadoleic |
20:1 (n-11) |
310.5 |
25 |
| cis-11-eicosenoic |
gondoic |
20:1 (n-9) |
310.5 |
– |
| cis-11-docosenoic | cetoleic | 22:1 (n-11) | 338.6 | |
| cis-13-docosenoic |
erucic |
22:1 (n-9) |
338.6 |
33.4 |
| cis-15-tetracosenoic |
nervonic |
24:1 (n-9) |
366.6 |
39 |
While this common fatty acid is mainly found acylated in glycerides, it may be found sometimes as ethyl esters in organs of animal treated with ethanol (Hungund BL et al., J Chem Pharmacol 1988, 37, 3001) and may serve as markers of ethanol intake. (Laposata M, Prog Lipid Res 1998, 37, 307).
The first synthesis of oleic acid appeared in the literature in 1934 (Noller CR et al., J Am Chem Soc 1934, 56, 1563).
Ethyl oleate was identified as a primer pheromone in honey bees in causing a delayed onset of foraging in younger individuals (Leoncini I et al., PNAS 2004, 101, 17559).
Palmitoleic acid was demonstrated to be an adipose tissue-derived hormone (a lipokine) that strongly stimulates in mice muscle insulin action and suppresses hepatosteatosis. Adipose tissue would be able to use lipokines to communicate with distant organs and regulate systemic metabolic homeostasis (Cao H et al., Cell 2008, 134, 933). The amount of palmitoleic acid in serum cholesterol ester reflects the hepatic pool of carbon flux from carbohydrates to fatty acids (Lands W et al., Am J Clin Nutr 1995, 61, 721). Furthermore, as a product of endogenous lipogenesis, palmitoleic acid is reported to correlate with indexes of adiposity and insulin concentrations (Kunesova M et al., Lipids 2002, 37, 27). On the other hand, palmitoleic acid has been used as a biomarker to trace dairy products intake in human (Mozaffarian D et al., Am J Clin Nutr 2013, 97, 854).
N-3 monoenes : An unusual isoform, the lauroleic acid (9-dodecenoic acid, 12:1 n-3) has been described as a natural metabolite of lauric acid (12:0) in rat hepatocytes (Legrand P et al., Lipids 2002, 37, 569)
N-5 monoenes : Seeds of Androsace septentrionalis (Primulaceae) were shown to contain an unusual fatty acid : 16:1 n-5 (Tsevegsuren N et al., Lipids 2003, 38, 1173).
N-7 monoenes : The 5-dodecenoic acid (denticetic acid, 12:1 n-7) is present in sperm whale oil. This compound was formerly known to be present in milk lipids.
The rare 21:4 n-7 has been described in some animal species (crab, sponge, protist), but it was found in high amount (about 9 % of the total fatty acids) in a opisthobranch mollusc, Scaphander lignarius (Vasskog T et al., Mar Drugs 2012, 10, 2676). That fatty acid was shown to be cytotoxic against human cancer cell lines normal lung fibroblasts, more than EPA and arachidonic acid.
N-8 monoenes : An isomer (12:1 n-8, linderic acid) has been found as the major fatty acid (47%) in the seed oil of a Lauraceae, Lindera umbellata (Hopkins CY et al., Lipids 1961, 1, 118). This fatty acid is not known to occur in any other plant family.
N-10 monoenes : Sapienic acid (16:1 n-10), a 16-carbon fatty acid with a single cis double bond at the sixth carbon from the carboxyl end, is the most abundant fatty acid in human wax sebum, and among hair-bearing animals is restricted to humans (Nicolaides N, Science 1974, 186, 19). Sapienic acid has been also identified in in human hair and nail samples beside 18:1n-10 (Destaillats F et al., J Chromatogr A 2011, 1218, 9384). Thus this fatty acid is truly unique to sebum, hair and nail and is not found anywhere else in the human body.
Notably, this fatty acid has been implicated in the pathogenesis of acne (Downing DT et al., J Am Acad Dermatol 1986, 14, 221) but was shown to be effective against gram-positive bacteria in human skin sebum (Wille JJ et al.., Skin Pharmacol Physiol 2003, 16, 176). Further works characterized its biosynthesis by a D-6 desaturase acting on palmitic acid (Ge L et al., J Invest Dermatol 2003, 120, 707).
N-15 fatty acids : An unusual 20 carbon fatty acid (20:1 n-15) is found in high concentration (about 60%) in seeds of Limnanthes alba (meadowfoam), an herbaceous winter annual plant native to the pacific Northwest area of the United States.
No fatty acid exists naturally with the double bond close to the terminal methyl group, only one is chemically synthesized from ricinoleic acid, the undecylenic acid (11:1 n-10).
One geometrical and several positional isomers of oleic acid exist with a trans double bond. Among the naturally occurring trans isomers, the double bond is in the (n-13), (n-12), (n-9) or (n-7) position.
Vegetable oils and fats are almost trans free, if not warmed at high temperature in the presence of active components. In contrast, trans fatty acids occur in most animal fats especially in butter and ruminant fats.
Among the most common, Elaidic acid (t9-octadecenoic acid) and t-vaccenic acid (t11-octadecenoic acid) are found in the rumen and in lipids of ruminant animals. trans-Vaccenic acid which is the major trans-monounsaturated fatty acid present in several food products (milk, yoghurt, cheese, butter and meats) results from the bio-hydrogenation of rumenic acid.
The elaidinization reaction was first obtained by a French pharmacist, Poutet JJE (Ann Chim Phys 1819, 12, 58), who observed that trioleine could be converted to the consistency of pork lard when treated with the oxides of nitrogen derived from mercurous nitrate (mainly nitrous acid). Later, Boudet F (Ann Chim Phys 1832, 50, 391; J Pharm 1832, 18, 469) studied accurately the reaction and isolated after saponification of "elaidine" (obtained from triolein) a fatty acid melting at 36°C which he named "acide élaidique" (elaïdic acid), from the Greek name of olive (elais, elaidos) .
Until 1952, elaidic acid was known only as a laboratory isomerization product of oleic acid. This trans fatty acid was demonstrated by infrared analysis to be present in substantial quantities in beef fat (Swern D et al., JAOCS 1952, 29, 44). Later, it was shown that trans fatty acids arise in the first stomach of ruminants as products of catalytic hydrogenation of dietary unsaturated fatty acids (conjugated by an isomerase) during bacterial fermentation. As a result, butter, cheese, milk, beef and mutton fats contain approximately 2-8% trans fatty acids by weight.
Trans-fatty acids are also formed in varying amounts during the industrial hydrogenation of plant or fish oils. This hydrogenation improves the thermal stability and prevents any oxidative process in very unsaturated oils. The natural kick found in cis-fatty acids disappears and the molecule becomes linear and thus has physical properties similar to saturated fatty acids as it was observed by Poutet in 1819.
Trans-fatty acids are also formed under the action of thiyl radicals.
Saturated fatty acids
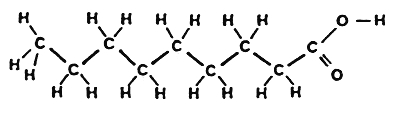
trans-Fatty acid
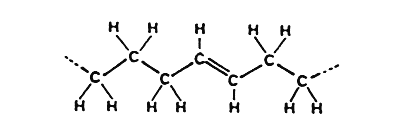
cis-Fatty acid
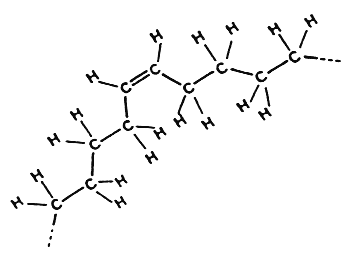
An unusual trans fatty acid, t3-hexadecenoic acid (trans-16:1 n-13), occurs in eukaryotic photosynthetic membranes (mainly in phosphatidylglycerol) from higher plants and green algae. As this fatty acid is absent from etiolated tissue, it has been inferred that it has a specific role associated with the light reactions of photosynthesis (Gounaris K et al., Biochem J 1986, 237, 313).
trans Fatty acids are formed by some bacteria (predominantly gram negative and under anaerobic conditions) via double-bond migration and isomerization.
The predominant 18:1 trans isomers in partially hydrogenated vegetal oils have their double bond in position t9, t10, t11 and t12, but their distribution (10-22% for t8, t9, t10, t11, t12 or t13 isomer) is distinct from that of milk fat, which contains (2-6% of the total fatty acids) vaccenic acid (t11-18:1) as the predominant isomer (about 60% of t11 and 4-8% for each of the others). The trans-18:1 acid contents of beef meat fat and tallow are about 2 % and 5 %, respectively. Its presence in ruminant fats is explained by a biohydrogenation of linoleic acid occurring in the rumen.
The trans isomers account for about 4.5 % of total fatty acids in ewe milk fat and 3 % in goat milk fat. While the contribution of these two milk sources may be estimated as negligible in most EEC countries, in Greece ewe and goat milk fat contribute for about 45 % of the daily consumption of vaccenic acid.
The daily per capita intake of trans-18:1 acids from ruminant fats was estimated to be about 1.5 g for people from most countries of the EEC, Spain and Portugal being exceptions (about 0.8 g/person/day) (review in Wolff RL, AOCS 1995, 72, 259). An estimation of the trans fatty acid content of foods and intake levels in France has been reported in 2007 (Laloux L et al., Eur J Lipid Sci Technol 2007, 109, 918). The trans fatty acid intakes and their food sources have been determined in the U.S. population (Kris-Etherton PM et al., Lipids 2012, 47, 931).
It is not yet known what effect continuous consumption and exposure to trans fatty acids isomers will have on human health, specifically those present in ruminant fats, deodorized vegetable oils, frying oils, and those present in synthesized products (Aldai N et al., Eur J Lipid Sci technol 2013, 115, 1378).
Vaccenic acid has been shown to attenuate complications observed in the metabolic syndrome, including dyslipidemia, fatty liver disease, and low-grade inflammation. An explanation of that property could be that vaccenic acid lowers intestinal inflammation by increasing the production of anandamide and related N-acylethanolamines (Jacome-Sosa M et al., J Lipid Res 2016, 57, 638).
A review of the diversity of adverse health effects of individual trans fatty acid isomers may be consulted (Gebauer SK et al., Lipids 2007, 42, 787). A review of the possible effects of trans fatty acids on heart health and the recommendations for the UK population has been reported (Denny AR, Nutr Bull 2008, 33, 124).
1 – Cyclopropane, cyclopropene, cyclopentyl,cyclopentenyl, cyclohexyl, and cyclohexenyl acids
Cyclopropane, cyclopropene
Some fatty acids contain either in the chain a cyclopropane ring (present mainly in bacterial lipids) or a cyclopropene ring (present in some seed oils), or at the end of the chain a cyclopentene ring (seed oils).
Among cyclopropane acids, lactobacillic acid (11,12-methylene-octadecanoic acid) is found mainly in a gram-negative bacteria, Lactobacillus arabinosus, where it was discovered in 1951 (Hofman K et al., J Biol Chem 1951, 195, 473). but also in protozoa and in the seed oil of Byrsocarpus coccineus (Connaraceae) (Spencer GF et al., Lipids 1979, 14, 72). Its ring has some properties of a double bond and it may be disrupted by acids or halogens. In bacteria, lactobacillic acid is never accompanied by cyclopropene acids.
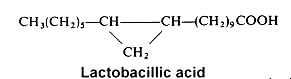
Cyclopropane fatty acids (mainly lactobacillic acid) were detected for the first time in milk (about 0.1% of milk fat) and they were present only in milk samples from cows fed with forages containing maize silage (Marseglia A et al., Food Chem 2013, 140, 711). Their determination can be proposed as a quality parameter of milk or feed.
The seeds of Litchi chinensis (Sapindaceae) from Reunion Island were shown to contain unusual amounts (35-48%) of an isomer of lactobacillic acid : cis-9,10-methylene-octadecanoic acid (Gontier E et al., Biochem Soc Trans 2000, 28, 578). That cyclopropane fatty acid is also present in longan (Euphoria longana) seed oil (Grondin I et al., Oléagineux Corps gras Lipides 1997, 4, 459). Furthermore, it was shown that this fatty acid was preferentially located at the sn-2 position of the triglyceride molecules.
Another cyclopropane fatty acid (9,10-methylene-hexadecanoic acid) was recently shown to be present in phospholipids of heart and liver mitochondria (Sakurada K et al., Biochim Biophys Acta 1999, 1437, 214). Its amount in bovine heart (about 4% of all fatty acids) is much greater than that in other tissues (less than 0.3%). The origin and the physiological role of these compounds remain unclear. Cyclopropane fatty acids have been also identified in human adipose tissue and serum (Sledzinski T et al., Lipids 2013, 48, 839). These compounds are stored in triacylglycerols but their origin remains unknown.
A novel brominated cyclopropyl fatty acid, majusculoic acid, with a 16-carbon chain has been reported in a cyanobacteria Lyngbya majuscula and was shown to exhibit antifungal activity against Candida albicans (MacMillan JB et al., J Nat Prod 2005, 68, 604).
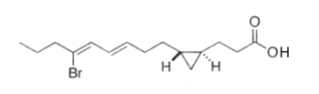
Two unsaturated cyclopropyl C28 fatty acids (named amphimic acids), 10,11-methylene-28:2(5Z,9E) and 10,11-methylene-28:3(5Z,9E,21Z) were isolated from an Australian sponge Amphimedon sp., and were shown to be DNA topoisomerase inhibitors (Nemoto T et al., Tetrahedron Lett, 1997, 38, 5667; Tetrahedron, 1997, 53, 16699).
Cyclopropene acids are found in Malvales seed oils (Sterculiaceae, Gnetaceae, Bombacaceae, Tiliaceae, Malvalaceae and Sapindaceae) and Baobab, Kapok and Mowrah seed oils which are used as human food in Madagascar. They can reduce the commercial value of cotton (Malvales, Gossypium) seed oil in interfering with animal fatty acid metabolism (desaturation of stearic acid). Such toxic acids are deactivated or removed by normal industrial treatments. The biological effects of these molecules have been reviewed by Andrianaivo-Rafehivola AA et al. (Oleagineux 1994, 49, 177).
Compounds containing cyclopropenoid ring are associated with several biological properties, such a : insecticide, antifungal, antibiotic, antiviral, hormonal, carcinogenic or antitumoral activities and enzyme inhibitor (Salaun J, Top Curr Chem 2000, 207,1).
The 9,10-methylene octadec-9-enoic acid was discovered by Nunn (1952) in Sterculia foetida oil. It was named sterculic acid by Schlenk et al. (J Am Chem Soc 1952,72, 500). An homologue molecule, 8,9-methylene heptadec-8-enoic acid, was discovered in 1957 by Mac Farlane (Nature 1957, 179, 830) in Malva verticillata seed oil and named malvalic acid. Cotton oil (Gossypium hirsutum) was the first oil, used for human consumption, where cyclopropenic acids were shown to be present (Coleman EC et al. 1972) by the Halphen test (J Pharm 1897, 390). Sterculic acid was shown to be an inhibitor of the D9-desaturase which converts stearic acid into oleic acid, altering membrane permeability and inhibiting cellular reproduction (Dewick PM, Medicinal natural products, J Wiley and Sons, NY, 2001).
Both cyclopropenic acids are present in Malvales in various proportions. In Sterculiaceae, malvalic acid is present in the range 3-26% and sterculic acid in the range 5-54%, in Malvaceae O.5-5% and 0.3-7%, in Gnetaceae 13-39% and 13-28%, and in Bombacaceae 2-20% and 1-46%, respectively.
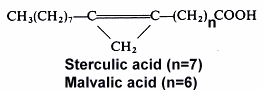
The Sterculia striata seeds, known as "chicha" nut in Brazil, are consumed by man and their oil was shown to contain about 4% of malvalic acid and 11% of sterculic acid (Aued-Pimentel S et al., J Chromatogr A 2004, 1054, 235). These levels may thus compromised the use of these nuts due to possible health implications caused by cyclopropenoid acid ingestion.
A review summarized the occurrence of cyclopropene acids in numerous plant families (Christie WW, in "Topics in Lipid chemistry", vol 1, pp1-50, Logos Press Ltd 1970). This review may be completed with that of Vickery JR (JAOCS 1980, 57, 87).
Cyclobutane acids (ladderanes)
Fatty acids with cyclobutane rings were discovered as constituents of membrane lipids in anaerobic ammonium-oxidizing (anammox) bacteria (Candidatus spp, order of the Planctomycetes) (Damsté S et al., Nature 2002, 419, 708). These fatty acids contain up to five linearly fused cyclobutane moieties, sometimes mixed with one or two cyclohexane rings (Damsté S et al., FEBS J 2005, 272, 4270). A typical example is pentacycloanammoxic acid which is composed of 5 fused cyclobutane units.
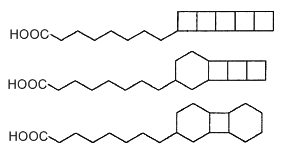
Ladderane fatty acids
Such "ladderane" structures are unprecedented in nature and occur in bacterial membranes as free fatty acids, fatty alcohols, alkyl glycerol mono- or di-ethers, phosphocholine diether and phosphocholine ester/ether and phosphoethanolamine diether and phosphoethanolamine ester/ether (Boumann HA et al., FEMS Microbiol Lett 2006, 258, 297). They are supposed to protect the cell from the toxic anammox intermediates located in specialized organelles (anammoxosomes). These lipids seem to be limited to these bacteria which were shown to play an important role in the nitrogen cycle in the ocean (Kuypers M et al., Nature 2003, 422, 608). Therefore, they are considered to be the unique biomarkers for the process of anaerobic ammonium oxidation. They were detected in suspended particulate matter obtained from various water depths in the Arabian Sea (Jaeschke A., Limnol Oceanogr 2007, 52, 780) and in sediments (Hopmans EC et al., Rapid Comm Mass Spectrom 2006, 20, 2099).
Cyclopentyl and Cyclopentenyl acids
Important cyclopentyl acids (tuberonic acid) and their glucosides belong to the jasmonate family that are likely to play a role in plant development (tuber-inducing factor in potatoes).
Among cyclopentenyl acids, Chaulmoogric acid is found in chaulmoogra oil from seeds of Flacourtiaceae (Hydnocarpus), which was used in folk medicine for treatment of leprosy. The chemical constitution of these fatty acids was first studied in 1904-1907 (Power FB, J Chem Soc 1907, 91, 563) but their structure was elucidated in 1925 (Shriner RL et al. Amer Chem Soc 1925, 47, 2727). The two main unsaturated cyclic acids, hydnocarpic and chaulmoogric acids, are predominant (from 9 to 75%) in seed oils of several plants of Flacourtiaceae family, but other analogues were described either with different chain length or with a double bond at various distance from the carboxylic group (Badami RC et al., Prog Lipid Res 1981, 19, 119). One of them, gorlic acid (n=12, a double bond between C6 and C7) is present at levels varying from 1.4 to 25% in seed oils.
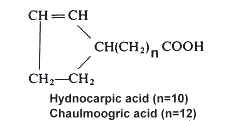
Study of the fatty acid compositions of five algae from the Solieriaceae (Gigartinales), Agardhiella tenera, Anatheca montagnei, Euchema cottonii, E. spinosum, and Meristotheca senegalensis, revealed the presence of two compounds similar to hydnocarpic and chaulmoogric acids but with a saturated cyclopentyl ring (Miralles J et al., Phytochemistry 1990, 29, 2161).
During heating of vegetal oils (refining or home-frying), it was shown that several cyclic fatty acid monomers are formed from linoleic and linolenic acids (Sebedio JL et al., Prog Lipid Res 1989, 28, 303). Thus, linolenic acid gives rise to sixteen dienoic acids containing a cyclopentenyl or a cyclohexenyl ring (Dobon G et al., J Chromatogr A 1996, 723, 349). Two examples of these derivatives are given below.
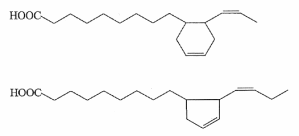
Cyclization and rearrangement mechanisms that are involved in the formation of cyclic fatty acids and their further transformation into bicyclic fatty acid monomers during the frying process have been determined. Insights into cyclization and rearrangement mechanisms that may be involved in the formation of these cyclic fatty acids have been reported by Destaillats F et al. (Destaillats F et al. Eur J Lipid Sci Technol 2005, 107, 767).
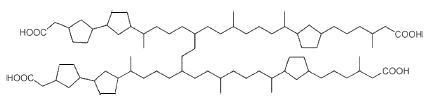
Naphthenic acids are complex mixtures of archaeal isoprenoid fatty acids formed by a link between two diacids with several cyclopentane rings (Lutnaes BF et al., Org Biomol Chem 2006, 4, 616). The structures of C80, C81 and C82 isoprenoid tetraacids have been finely described (Lutnaes BF et al., Org Biomol Chem 2007, 5, 1873). These tetra-acids (Arn acids) are present in crude oil and their calcium salts can cause serious problems during the industrial process (corrosion, formation of emulsion, clogging by the salts). The family members differ by their number of cyclopentane rings and by their molecular weight (400-1300 g/mol). The most abundant Arn acid has 80 carbon atoms and 6 cyclopentane rings.
Cyclohexyl and cyclohexenyl acids
Cyclohexyl fatty acids were first found in butter (Shogt JC et al., 1965, 6, 466) and then in sheep fat (Hansen RP et al., J Sci Food Agric 1967, 18, 225) and rumen bacteria (Hansen RP, Chem Ind 1967, 39). The most common cyclohexyl acids are 11-cyclohexyl-C11 and 13-cyclohexyl-C13 (see below).
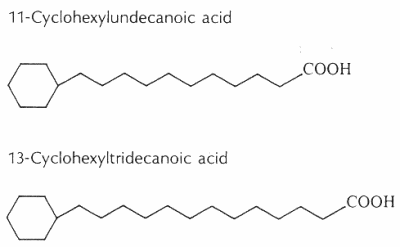
The content of w-cyclohexyltridecanoic acid varied from 0.0% to 0.15% of milk fat, and it was higher in milk samples from cows fed with diets richer in cereal meals (Marseglia A et al., Food Chem 2013, 140, 711).
While these w-cyclohexyl acids were detected in a mesophile bacteria Curtobacterium pusillum (Kawagushi A et al., J Biochem 1986, 99, 1735), they are curiously more abundant in thermophile bacteria. Thus, they are, in equal amounts, the major components (about 65%) of the fatty acids in Bacillus acidocaldarius (= Alicyclobacillus), a gram-positive rod-shaped prokaryote found in acid hot springs up to 65°C (De Rosa M et al., Chem Commm 1971, 1334). They were also found in ten strains of acido-thermophilic bacteria isolated from different Japanese hot springs (Oshima M et al., J Biol Chem 1975, 250, 6963). These fatty acids were found in the esterified form in glyceride type lipids, amounted to 74 to 93% of the total fatty acids in the bacteria, and were shown to be formed from glucose and shikimic acid. More recently, cyclohexyl undecanoic acid was shown to be the major cellular fatty acid in Propionibacterium cyclohexanicum, a new acid-tolerant bacteria isolated from spoiled orange juice (Kusano K et al., Int J Syst Bacteriol 1997, 47, 825).
Some C17 and C19 cyclohexyl alkanoic acids have been identified in acidothermophilic bacteria in hot springs. Furthermore, a series of alkyl cyclohexanes and cyclohexyl alkanoic acids were detected in lacustrine saline petroleum samples from Brazil (Nascimento LR et al., Org Geochem 1999, 30, 1175). The origin of these compounds is still unknown, but a correlation was made with the presence of Alicyclobacillus in the formation water and oil samples (Rodrigues DC et al., Org Geochem 2005, 36, 1443).
During heating of vegetal oils, linolenic acid gives rise to dienoic acids containing a cyclohexenyl or a cyclopentenyl ring. Similarly, deodorization of fish oil rich in eicosapentaenoic and hexaenoic acids generates about 15 cyclohexyl and cyclopentyl fatty acids with a 20 and 22 carbon chain, respectively (Berdeaux O et al., J Chromatogr A 2007, 1138, 216). One of the most abundant cyclic monomers formed from EPA (8-(2′-hexylcyclohexyl) octanoic acid) is shown below.
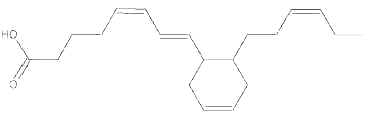
8-(2′-hexylcyclohexyl) octanoic acid
A more complex fatty acid containing a highly functionalized cyclohexanone has been isolated from the endophytic fungi Pestalotiopsis spp and Monochaetia sp living in the thalli of lichens (Li JY et al., Phytochemistry 2001, 56, 463). The compound, named ambuic acid, contains a hexa-substituted cyclohexanone moiety, as it was found in a tetracycline antibiotic.
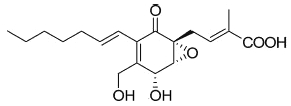
Ambuic acid
Ambuic acid has displayed antifungic activity against human pathogenic fungi. Several secondary metabolites of ambuic acid have been described in the same fungus (Pestalotiopsis sp) (Ding G et al., J Nat Prod 2009, 72, 182). Ambuic acid and one of its metabolites displayed anti-microbial activity against the Gram-positive bacterium Staphylococcus aureus.
Furan fatty acids : These fatty acids contain a furan ring in the chain. They were found for the first time in a seed oil of Exocarpus cupressiformis (Santalaceae) (Morris LJ et al., Tetrahedron Lett 1966, 36, 4249).
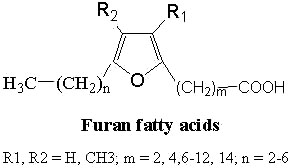
The furan acid described initially was the 9,12-epoxy-octadeca-9,11-dienoic acid (R1, R2 = H, m = 7 and n = 5 in the general formula above).
A common nomenclature describing these fatty acids (as F1, F2,…) is used. This naming originated from elution order in gas chromatography.
The most frequent compound is known as F6 (12,15-epoxy-13,14-dimethyleicosa-12,14-dienoic acid) while, later, two series of propyl- and pentyl-substituted F-acids in the 5-position of the furan ring was described in fish, crayfish, soft coral and various algae and terrestrial plants. Their presence was more recently detected in mammals including man (Puchta V et al., Liebigs Ann Chem 1988, 25). In man, furan fatty acids were located in phospholipid fractions.
In fish, furan fatty acids are found in liver cholesterol esters and in testis triglycerides, but are also present in phospholipids. In 1977, height furan fatty acids were listed in several organs of 20 fresh water fish species (Glass RL et al., Lipids 1977, 12, 828). Forty furan fatty acids were identified in a marine fish oil, two propyl-substituted molecules being reported for the first time (Wahl HG et al., J High Resol Chromatogr 1994, 17, 308).
As a furan fatty acid (10,13-epoxy-11-methyloctadeca-10, 12-dienoic acid) was detected in the cellular lipids of several marine bacteria (Shirasaka N et al., Biochim Biophys Acta 1995, 1258, 225), the authors propose that those detected in marine fish are derived from marine plants and/or intestinal bacteria of fishes.
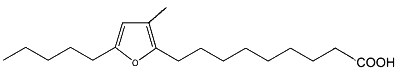
10,13-Epoxy-11-methyloctadeca-10, 12-dienoic acid
Three new furan derivatives (plakorsins A-C) were isolated from the Taiwanese marine sponge Plakortis simplex, n being equal to 15 and m being equal to 1. These fatty acids exhibited cytotoxic activity against cultured cells (Shen YC et al., J Nat Prod 2001, 64, 324). Furan acids were found in 10 genera of Gorgonaria corals without zooxanthellae (endosymbiotic microalgae of the genus Symbiodinium). Furan acids were not found in Gorgonaria with zooxanthellae, alcyonaria and other soft corals. A possible source of furan acids in Gorgonaria might have been microalgae occurring in food or bacteria and microalgae associated with the corals (Imbs AB et al., Chem Nat Compounds 2009, 45, 898).
Due to the analytical difficulties in their determination furan fatty acids have been scarcely determined in food. There was an early report of the presence of a furan acid in latex rubber from Hevea brasiliensis (Hasma H et al., Lipids 1978, 13, 905). This was confirmed by the identification of a furan fatty acid (10,13- epoxy-11-methyl octadeca-10,12 dienoic acid) esterified in latex glycoglycerolipids (Liengprayoon S et al., Phytochemistry 2011, 72, 1902). Other vegetal sources were described. Thus, several furan species were found in grasses (240 m
g/g dry w.), lemon (30 mg/g dry w.), strawberry, orange, potato (2-5 mg/g dry w.) and even in mushroom (160 mg/g dry w.) (Hannemann K et al., Lipids 1989, 24, 296). By far the richest source of furan fatty acids in food is fish. Concentrations of up to 4 g/100 g oil were reported in the literature (Vetter W et al., J. AOCS 2012, 89, 1501). Frequently, the furan acid concentrations were in the range 0.1-0.9 g/100 g oil, with a clear dominance of dimethylated derivatives. Lower concentrations have been reported in other fatty sources : butter (48 mg/100 g), soybean oil (42 mg/100 g), wheat germ oil (22 mg/100 g).
An extensive review of the occurrence of furan fatty acids may be found (Spiteller G, Lipids 2005, 40, 755). A review of their importance in food may be also consulted (Vetter W et al., Lipid technol 2013, 25, 7).
It appears possible that these furan compounds may serve as antioxidants in biological systems through their ability to scavenge free radicals (Okada Y et al., Biol Pharm Bull 1996, 19, 1607). The radical scavenging Furan acids are able to eliminate radicals before their concentrations are exponentially increasing by means of a radical chain reaction. The strong capability of these compounds to serve as radical scavengers suggests that plants and algae can use these compounds to defend against the deleterious effect of UV radiation (Spiteller G, Lipids 2005, 40, 755). A review has focused mainly on the functions and roles of furan acids in plants (Mawlong I et al., Phytochem Rev 2016, 15, 121). It is recalled that In plants, they are bound to phospholipids by substituting polyunsaturated fatty acids and function as free radical scavengers suggesting their role in defense against oxidative stress.
It was hypothesized that the radical-scavenging ability of furan fatty acids could contribute to the protective properties of fish diets from heart disease (Spiteller G, Lipids 2005, 40, 755). A first in vivo demonstration has been given using furan fatty acids isolated from a mussel Perna canaliculus, native to the New Zealand coast (Wakimoto T. et al., PNAS 2011, 108, 17533). These fatty acids exhibited in a rat model of adjuvant-induced arthritis a more potent anti-inflammatory activity than that of EPA. These anti-inflammatory effects corroborate the observation of a lower incidence of arthritis in coastal Maoris, eating these mussels, compared with its prevalence in European or inland Maori people.
A rapid method for the preparation of C18 furanoid fatty aster from methyl ricinoleate involving dry-column chromatography was described (Lie Ken Jie MSF et al., JAOCS 1981, p. 705).
A method for the quantitative determination of furan acids in food has been reported (Vetter W et al., JAOCS 2012, 89, 1501). After extraction, transesterification and silver ion chromatography, the furan acids are anayzed by GC/EI-MS.
Epoxy acids
Epoxy acids are present in a number of seed oils (in triacylglycerols) and in plant cutin and suberin. The natural species are all C18 compounds, saturated on unsaturated. On prolonged storage, they can be formed as hydroxy derivatives. For example, 9,10-epoxystearic and 9,10-epoxyoctadec-12-enoic (coronaric acid) acids are found in sunflower seeds (Chrysanthemum). The seed oil of Bernardia pulchella (Euphorbiaceae) contains 91% of coronaric acid (Spitzer V et al., JAOCS, 1996, 73, 1733). These fatty acids are commonly used to make paints and coatings.
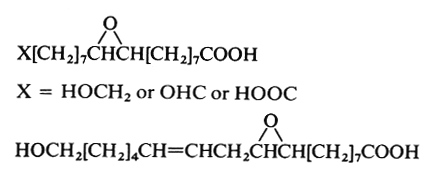
These two epoxy acids are commonly present in cutin and suberin up to an amount of 34% of the lipid content.
Vernonia and Stokesia species are among plants identified as containing epoxy fatty acids at high percentages in their seed oils. Works are in progress in Africa to improve crops of these plants.
Vernolic acid is the best known epoxy acid from natural sources. This isomer of coronaric acid is 12,13-epoxy-9-octadecenoic acid was characterized as an epoxy oleic acid (Gunstone FD, J Chem Soc 1954, 1611) and was shown to be abundant in seed oils of the Compositae Vernonia sp as well as Euphorbia lagascae (from 60 up to 75%).
Small quantities (about 2.5%) of 9,10-epoxy-18:0, vernolic acid and coronaric acid were found in peanut (Arachis hypogaea) germplasm (Hammond EG et al., JAOCS, 1997, 74, 1235). Vernolic acid was also shown to be present (7%) in the seed oil of Geranium sanguineum (Geraniaceae) (Tsevegsuren N et al. Lipids 2004, 39, 571).
A C20 homologue of vernolic acid has been found in Alchornea cordifolia (Euphorbiaceae) seed oil (Kleiman R et al., Lipids 1977, 12, 610). This new fatty acid, cis-14,15-epoxy-cis-11-eicosenoic acid has been named as alchornic acid.
Epoxide groups are also present in bacterial lipids.
Epoxides may be also formed during peroxidation attack of unsaturated fatty acids. This chemical reaction (with peroxyformic acid) is used to transform vegetal oils (mainly canola) into bio-resins used in composite boards and in polyurethane adhesive and insulating foam applications (Kong X et al., Lipid Technol 2012, 24, 7).
In living organisms, coronaric and vernolic acids, known also as leukotoxins, were shown to be formed in lung, vascular system, and neutrophils by cytochrome P450 enzymes (epoxydases) interfering in the regulation of vascular tone, homeostasis, and blood pressure. Several studies suggested that these epoxides have toxic cardiovascular effects which may result in death at high doses. Their strong toxicity seems to be related to the disruption of the endothelial barrier function and a modulation of any dysfunction mediated by various contaminants (Slim R et al., Toxicol Appl Pharmacol 2001, 171, 184). Leukotoxins have been suggested to be a toxic mediator ("burn toxin") causing acute respiratory distress syndrome in burn patients (Hayakawa M et al., Biochem Int 1990, 21, 573). Further studies have demonstrated that leukotoxin-diol, the hydrated product of leukotoxin, is even more toxic that the parent leukotoxin in vitro (Moghaddam MF et al., Nature Med, 1997, 3, 562) and in vivo (Zheng J et al., Am J Respir Cell Mol Biol 2001, 25, 434). Thus, these by-products are the putative toxic mediators involved in the development of acute respiratory distress syndrome.
Epoxides of arachidonic acid, EPA and DHA are also formed by cytochrome P450 and are very potent bioactive molecules.
Hydroxy derivatives of epoxy-arachidonic acid (hepoxilins) are described with the bioactive lipoxygenase products.
Cyclic fatty acid endoperoxides
It is well known that cyclic peroxide products may be produced during the autoxidation of polyunsaturated fatty acids. In contrast with these labile compounds, several cyclic peroxides have been described in lipid extracts from marine sponges (Faulkner DJ, J Nat Prod 1997, 14, 259). Almost all these molecules are assumed to derive from the polyketide pathway and derived from a common backbone : CH3-(CO-CH2)n-COOH
The first of these compounds to be reported was plakortin, a six membered ring cycloperoxide found in 1978 by Faulkner’s group in the marine sponge Plakortis halicondrioides (Higgs M D et al., J Org Chem 1978, 43, 3454).
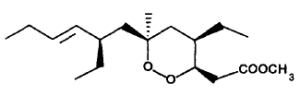
Subsequently, a series of related bioactive metabolites have been isolated. They include plakinic acids (Davidson, B. S. J. Org. Chem., 1991, 56, 6722), the strongly antifungal peroxyplakoric acids (Kobayashi M et al., Pharm Bull, 1993, 41, 1324) and the recently reported activators of cardiac Ca ATPase plakortones A-D (Patti AD et al., Tetrahedron 1996, 52, 377).
In 1998, several cyclic peroxide homologues have been described in the same sponge species, either active against cancer cell lines (Harrison B et al., J Nat Prod 1998, 61, 1033), toxic toward Artemia larvae (Braekman JC et al., J Nat Prod 1998, 61, 1038), inhibitor of the protozoan causing leishmaniasis (Compagnone RS et al., Tetrahedron 1998, 54, 3057), or without known biological activity (Fontana A et al., J Nat Prod 1998, 61, 1427). Several metabolites of plakortin with various cytotoxic activities have been also isolated from Plakortis simplex : dihydroplakortin and two dodecanoic derivatives (Cafieri F et al., Tetrahedron 1999, 55, 7045). Later, other related cytotoxic endoperoxides have been isolated from the Okinawan sponge Plakortis lita and named Haterumadioxins (Takada N et al., J Nat Prod 2001, 64, 356) and from Plakortis simplex (Holzwarth M et al., J Nat Prod 2005, 68, 759).
This compound was first considered as a microbial growth factor but it was found not only in yeast but also in beef liver from which it was first isolated in pure form (Reed LJ et al., J Am Chem Soc 1953, 75, 1267). This isolation is one of the more spectacular achievements in the purification of natural products, since 30 mg of crystalline lipoic acid was obtained from 10 tons of liver. Lipoic acid was named also thioctic acid or 1,2-dithiolane-3-pentanoic acid. After its absorption, this acid is reduced enzymatically by NADH or DADPH to dihydrolipoic acid (or 6,8-dithiane octanoic acid) in various tissues. A fraction of the total lipoic acid pool is covalently bound to enzyme molecules through an amide linkage with a lysine residue.
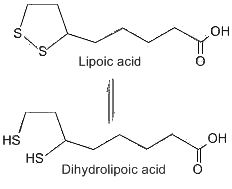
First shown necessary for bacteria, lipoic acid was demonstrated to be a coenzyme in the glycine cleavage system and in the dehydrogenase complex. Now, lipoic acid is considered as an efficient antioxidant since with its reduced form it constitutes a redox couple via modulation of NADH/NAD ratio. Consequently, lipoic acid has gained a special interest as a therapeutic agent (Packer L et al., Free Radic Biol Med 1997, 22, 359). It can scavenge hydroxyl and peroxyl radicals but also chelates transition metals (Fe, Cu…). Packer L. considered that lipoic acid is perhaps the most powerful of all the antioxidants, it may offer an efficient protection against many heart diseases, it is currently used to relieve the complications of diabetes (Packer L et al. The antioxidant miracle. John Wiley & Sons 1999).
This class, known also as ethynoic acids, includes fatty acids which contain a triple bond (or more as in polyacetylenes) and eventually one or two double bonds. While many acetylenic fatty acids have been prepared synthetically, only some species are found in natural oils.
MONOACETYLENIC ACIDS
Acetylenic fatty acids (tariric acid) were discovered in the seed fat of Picramnia Sow (Simarubaceae) in the 19th century by the French chemist Arnaud A (Bull soc chim 1892, 7, 233). They appear to be common in tropical plants mainly belonging to the order of Santalales including the 2 families Santalaceae and Olacaceae. These fatty acids form up to 90% or 50% of the seed fat of the Santalaceae or Olacaceae, respectively.
We give below some of the best known acetylenic fatty acids found in plants:
Stearolic acid (9-octadecynoic acid) is present in seeds of Santalaceae.
Tariric acid (6-octadecynoic acid) was first reported in the seed fat of the Simarubiaceae Picramnia tariri (Arnaud A, C R Acad Sci 1892, 114, 79) an later in other Picramnia species. Among them, the seed oil from Picramnia sow, a plant indigenous to Guatemala, has been reported to contain nearly 95% of tariric acid (Steger A et al., Rec Trav Chim 1933, 52, 593).
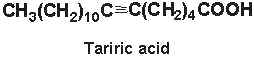
Santalbic acid (or Ximenynic acid) has been described in the seed fat of Santalum album at high concentration (50%) (Madhuranah MK et al., J Indian Chem Soc 1938, 15, 389) and its structure was later identified (Gunstone FD et al., Chem Ind 1954, 1112). 
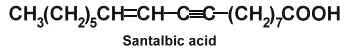
The two acetylenic fatty acids, santalbic acid and stearolic acid, were isolated from the seeds of Exocarpus and Santalum (Morris LJ et al., Chem Ind 1966, 12, 460). This distinctive biochemical feature was discovered in santalwood oil from a small tree or shrub (Santalum spicatum) native to the Western Australia (Hatt HA et al., J Sci Food Agric 1956, 7, 130). Its large fruit contain a hard-shelled seed rich in a drying fixed oil (50-60%). About 30-35% of total fatty acids are represented by santalbic acid and 1% by stearolic acid. When administered to animals these fatty acids were described to inhibit several lipoenzymes (lipoxygenase, prostaglandin synthetase) and modify tissue fatty acid composition (Liu Y et al., Lipids 1997, 32, 965).
Two acetylenic acids (6-octadecynoic and 6-nonadecynoic acids) were described in the roots of a Peruvian plant (Pentagonia gigantifolia, Rubiaceae) and were shown to inhibit the growth of fluconazole-susceptible and -resistant Candida albicans strains (Li XC et al., J Nat Prod 2003, 66, 1132). Their antifungal potencies were reported to be comparable to those of amphotericin B and fluconazole and to have low cytotoxicity.
6,9-octadecenynoic acid (6-octadecen-9-ynoic acid) was isolated from nuts of Ongokea klaineana. This compound contains a triple-bond at carbon 9 and a double bond at carbon 6.
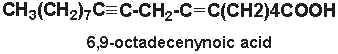
Pyrulic acid (t10-heptadecen-8-ynoic acid) was isolated first from seed oil from a Santalaceae Pyrularia pubera, and recently found in a Olacaceae from southern Brazil, Heisteria silvanii (Spitzer V et al., Lipids 1997, 32, 1189).
Crepenynic acid (9-octadecen-12-ynoic acid) was found in high concentration (60%) in oil extract from seeds of Crepis foetida (Compositae) (Mikolajczak KL et al., J Org Chem 1964, 29, 318). Toxic effects of plants containing this fatty acid have been reported in Australian sheep.
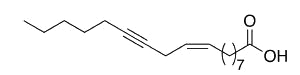
Crepenynic acid
The distribution of crepenynic acid and dehydrocrepenynic acid (9,14-octadecen-12-ynoic acid) (65% of total fatty acids) in triacylglycerols of the aril and cotyledon oils of Afzelia cuanzensis (Caesalpinaceae) was studied by nuclear magnetic resonance spectroscopy (Vlahov G, Phytochemistry, 1996, 42, 621).
The oil from the seed of Alvardoa amorphoides (Simarubiaceae) was reported to contain about 15% of 17-octadec-6-ynoic acid, the predominant fatty acid of this oil being tariric acid (Pearl MB et al., Lipids 1973, 8, 1284).
Similar acetylenic acids, but with 14 to 18 carbon atoms and one to three hydroxyl groups have been described in the fungus Coriolopsis gallica (basidiomycete) (Zhou ZY et al., J Nat Prod 2008, 71, 223).
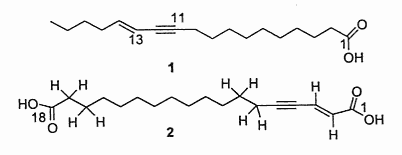
Two acetylenic acids with one triple double bond and one double bond were described in a Santalaceae, Nanodes muscosa from Chili. One of them (compound 2) constitutes the first example of an a,w fatty diacid with a conjugated enzyme system from a natural source (El-Jaber N et al., J Nat Prod 2003, 66, 722).
Scleropyric acid was isolated from another Santalaceae, Scleropyrum wallichianum (Suksamrarn A et al., Chem Pharm Bull 2005, 53, 1327). That acetylenic acid exhibited antimycobacterial (MIC : 25 mg/ml) and antiplasmodial (MIC : 7.2 mg/ml) activities.
![]()
Scleropyric acid
An acetylenic fatty acid with ene-yne-ene structure was described in an Olacaceae, Heisteria silvanii (popular name "casca-de-tatu): heisteric acid (Spitzer V et al., Lipids 1997, 32, 1189). It has been tentatively characterized by their mass spectra as cis-7,t11-octadecen-9-ynoic acid and determined to present at a level of about 23% in seed oil
![]()
A conjugated t,t-diunsaturated acetylenic acid was found as a main component in the seed oil of an ornemental Asteraceae, Tanacetum (Chrysanthemum) corymbosum (Compositae). This fatty acid was shown to be t8, t10-octadecadien-12-ynoic acid (Tsevegsuren N et al., Lipids 1998, 33, 723).
Acetylenic acids were also described in sponges. Several compounds with a branched-chain, an acetylenic moiety in the middle of the molecule and a terminal double bond, and sometimes with a methoxy group were described in a marine sponge Stelletta species (Lee HS et al., J Nat Prod 2003, 66, 566; Zhao Q et al., J Nat Prod 2003, 66, 408).
POLYACETYLENIC ACIDS
Polyacetylene fatty acids are not commonly found in living organisms. Mycomycin, which is produced by a mold-like Actinomycete and is active against the Bacilli sp of human tuberculosis was discovered in 1947 by Johnson EA et al. (J Bacteriol 1947, 54, 281). This fatty acid has two triple bonds but has also an allenic structure : tridecatetra-3,5,7,8-en-10,12-diynoic acid.
A diynoic acid (13,14-dihydrooropheic acid) has been isolated from an Indonesian plant (Mitrephora celebica, Annonaceae) and was shown to have a potent antimicrobial activity (Zgoda J et al., J Nat Prod 2001, 64, 1348).
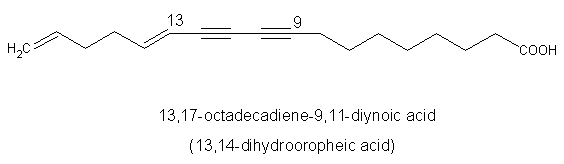
Another diynoic acid (octadecen-13-en-9,11-diynoic acid) has been described in Santalum acuminatum and also in Nanodea muscosa (Santalaceae) where its exact structure was elucidated (El-Jaber N et al., J Nat Prod 2003, 66, 722). Several others have been described in Olacaceae : octadeca-13-en-9,11-diynoic acid (exocarpic acid), octadeca-13,17-dien-9,11-diynoic acid, octadeca-17-en-9,11-diynoic acid (isanic acid), and octadeca-9,11-diynoic acid (Badami RC et al., Prog Lipid Res 1981, 19, 119).
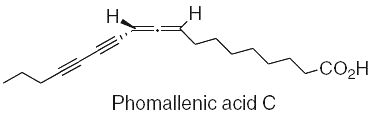
Three fatty acids with an allenyldiyne structure, phomallenic acids A-C, have been isolated from the fermentation broth of Phoma sp. Phomallenic acid C had the most potent activity as an inhibitor of bacterial FAS II pathway (Ondeyka JG et al., J Nat prod 2006, 69, 377).
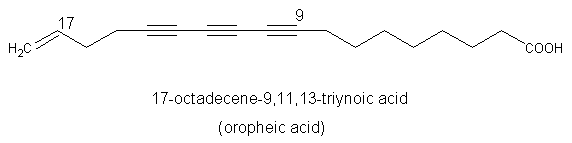
A triynoic acid (oropheic acid) was isolated from leaves of Orophea enneandra (Annonaceae)(Cavin A et al., J Nat Prod 1998, 61, 1497) and of Mitrephora celebica (Zgoda J et al., J Nat Prod 2001, 64, 1348). It displayed a significant activity against the fungus Cladosporium.
A 9-carbon triynoic acid containing a terminal acetylene has been isolated first from the Basidiomycetes Psilocybe sarcocephala (Jones ERH, Proc Chem Soc 1960, 199) but was later found in other fungi of the same group. An amide of that fatty acid with p-aminobenzoic acid has been shown to be present in the fungus Baeospora myosura and to have a selective antibacterial activity (Parish CA et al., J Nat Prod 2004, 67, 1900). Triacetylenic acids, named corticatic acids, have been isolated from the marine sponge Petrosia corticata. One of them is shown below. All are geranylgeranyltransferase type I (from Candida albicans) inhibitors (Nishimura S et al., J Nat Prod 2002, 65, 1353).
Corticatic acid A
Another triynoic acid with 20 carbon atoms, from synthetic origin, is frequently used to experimentally inhibit essential fatty acid metabolism and especially cyclooxygenase and lipoxygenase pathways: 5,8,11,14-eicosatetraynoic acid (ETYA).
A tetraynoic acid (Haliclonyne) was isolated from the marine sponge Haliclona collected at Eilat (Israel) (Chill L et al., J Nat Prod 2000, 63, 523). This highly polyacetylene compound is a C47 oxo-octahydroxy-dientetrayne carboxylic acid.
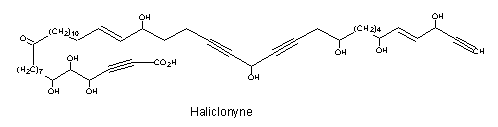
A review of several polyacetylenic acids in plants and the current state of knowledge of the biochemistry and molecular genetics of polyacetylenic metabolic pathways may be consulted (Minto RE et al., Prog Lipid Res 2008, 47, 233).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire