
SESQUITERPENES
Sesquiterpenoids are defined as the group of 15 carbon compounds derived by the assembly of 3 isoprenoid units and they are found mainly in higher plants but also in invertebrates. Sesquiterpenes, with monoterpenes, are an important constituent of essential oils in plants. They are the most diverse group of isoprenoids. In plants, they function as pheromones and juvenile hormones.
Sesquiterpene structures present several acyclic, mono-, bi-, tri-, and tetracyclic systems. Some of natural sesquiterpenoids are shown below.
Acyclic compounds
The acyclic representative are also called farnesans, term derived from the basic structure, farnesol. Farnesol and nerolidol are very common and are isolated from essential oils of various sources.
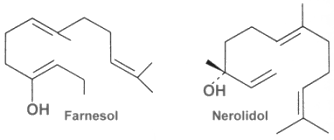
Farnesol is widely distributed in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, and balsam. It is used in perfumery to emphasize the odors of perfumes. Moreover, it is a natural pesticide for mites and is also a pheromone for several insects and mammals, including elephants (teritorial marking, individual recognition, mate attraction). Farnesyl acetate is found in the web of some spiders (Pholcidae) to attract females (Schulz S, J Chem Ecol 2013, 39, 1). Farnesol was also show to be the âquorum-sensing moleculeâ identified in fungi (Hornby JM et al., Appl Environ Microbiol 2001, 67, 2982). The presence of farnesol prevents the yeast-to-mycelium conversion, resulting in actively budding yeasts without influencing cellular growth rates. This study is the first to identify an extracellular molecule mediating an eukaryotic quorum-sensing system.
Farnesol is active against a variety of Candida albicans at concentrations between 1 and 50 mM (Hornby JM et al., Antimicrob Agents Chemother 2003, 47,2366).
Farnesol is frequently esterified with one fatty acid having 8 to 12 carbon atoms.
Farnesene, an analogue of farnesol, is a collective name that refers to four stereoisomers of alpha-farnesene and two stereoisomers of beta-farnesene. It is known to act as an alarm pheromone in aphids. Released during predator attack, it causes aphids to stop feeding, disperse, and give birth to winged (rather than wingless) forms, which leave their host plants. Farnesene is produced by de novo biosynthesis by cotton plants when damaged by insect herbivories (Paré PW et al., Plant Physiol 1997, 114, 1161). These compounds likely mediate the interaction between herbivores and their natural enemies, attracted by terpenes.
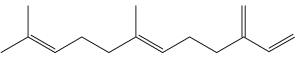
Farnesene
Microbially produced farnesene has been used as a precursor of polymers for the tire industry and the fully hydrogenated compound, farnesane, has been evaluated as a possible diesel additive. Amyris has produced the
fuel Biofene® derived by chemical hydrogenation of farnesene.
Nerolidol is present in neroli, ginger, jasmine, lavender, tea tree and lemon grass. The aroma of nerolidol is woody and reminiscent of fresh bark. It is used as a flavoring agent and in perfumery. It was also shown to be produced by the leaves of a large number of plant species in response to herbivory insects and then to be transformed into a C11-homoterpene (4,8-dimethyl-1,3,7-nonatriene) which attracts predatory insects (Dicke M et al., J Chem Ecol 1990, 16: 3091â3118).
4,8-Dimethyl-1,3,7-nonatriene
Among the acyclic species, two compounds are well known for their importance in invertebrate endocrinology. One, methyl farnesoate is now considered as the crustacean juvenile hormone (Homola E et al., Comp Biochem Physiol 1997, 117B, 347) and is synthesized by the mandibular organs and is present in the hemolymph. Its structure is nearly identical to the other one, the insect juvenile hormone III which regulates metamorphosis and reproduction. Both are synthesized from farnesoic acid in the corpora allata.
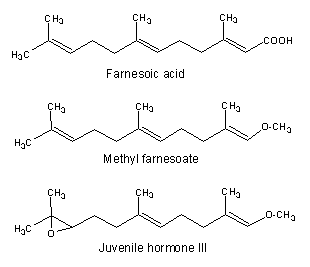
Farnesoic acid has been determined to be the autoregulatory substance involved in the regulation of the morphological transition of the yeast Candida albicans between a budding form and a multicellular invasive filamentous form (Oh KB et al., Proc Natl Acad Sci USA 2001, 98, 4664). This transformation has been postulated to contribute to the virulence of this organism. These findings might have medicinal value in the development of anti-fungal therapies.
Cyclic compounds
Abscisic acid plays a key role in plants in the regulation of stomatal closure by regulating ion channel activities and water exchanges across the plasma membrane of guard cells.
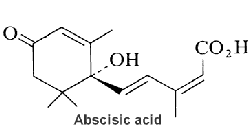
Cyclic ADP-ribose (cADPR) has been shown to mediate signaling of abscisic acid in the drought-stress response leading to activation of gene transcription and to stomatal closure (Leckie C P et al., Proc Natl Acad Sci U S A 1998, 95, 15837). It was shown that diacylglycerol pyrophosphate plays a role as phospholipid second messenger in abscisic signaling (Zalejski C et al., Plant J 2005, 42,145). A review of the signaling network may be found in a paper by Giraudat J (Curr Opin Cell Biol 1995, 7, 232)and in the "Plant hormones" textbook (Litwack G Ed, Elsevier, 2005). Abscisic acid is an end product of neoxanthin or violaxanthine peroxidation and reduction giving an apocarotenal (apocarotenoid) with a short side chain (5 carbons), followed by a final oxidation into an acid form (Seo M et al., Trends Plant Sci 2002, 7, 41).
Abscisic acid has also a variety of roles in plant development, bud and seed dormancy, germination, cell division and movement. It induces also storage protein synthesis in seeds and may be involved in defense against insect attack. Abscisic acid is biosynthesized via carotenoids (zeaxanthin, neoxanthin, violaxanthin) in roots and mature leaves. Its direct precursor is xanthoxin which is a natural inhibitor of plant growth. Abscisic acid is ubiquitous in lower and higher plants, it is present also in algae. Only the C2-cis, C4-trans isomer is biologically active. A mechanism of abscisic signaling in connection with cyclic ADP-ribose and calcium movement has been demonstrated to mediate temperature signaling in sponges (Zocchi E et al., Proc Natl Acad Sci USA 2001, 98, 14859) as well as tissue regeneration in Cnidaria (Puce S et al., J Biol Chem 2004, 279, 39783).
Abscisic acid is not restricted to the plant kingdom and primitive invertebrates since it has been shown to be present in the central nervous system of pigs and rats (Le Page-Degivry MT et al., Proc Natl Acad Sci USA 1986, 83, 1155). Evidence was provided that it is also involved in the stimulation of human granulocytes with cyclic ADP-ribose as second messenger (Bruzzone S et al., Proc Natl Acad Sci USA 2007, 104, 5759). This lipid may be considered as a new pro-inflammatory cytokine in humans. Human granulocytes and smooth muscle cells are also functionally activated by abscissic acid (Magnone M et al., J Biol Chem 2009, 284, 17808). That messenger can be considered as a new signal molecule involved in the development of atherosclerosis.
Abscisic acid was shown to be a endogenous stimulator of insulin release from human pancreatic b cells with cyclic ADP-ribose as second messenger (Bruzzone S et al., J Biol Chem 2008, 283, 32188). This observation suggests that this lipid phytohormone may be involved in the physiology of insulin release, mainly in its dysregulation under conditions of inflammation. Together with jasmonates, abscisic acid was shown to control excitability and closure of the insect trap of Dionaea muscipula (Venus flytrap) (Escalante-Perez et al., PNAS 2011, 108, 15492).
Cadalene has the cadinane skeleton and is present in essential oils and in many plants. It is used as a biomarker in paleobotanic studies. In connection with retene (1-methyl-7-isopropyl phenanthrene), it enables the estimation in sediments of the importance of Pinaceae in ancient forests.
Some important sesquiterpenes
|
|
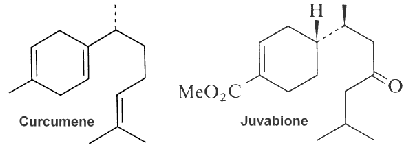
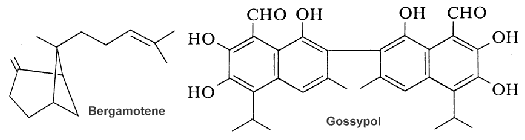
The function of curcumene as insecticide, repellent, and insect feeding deterrent has been previously reported. A parent compound, bisabolol, isolated from essential oils of a variety of plants, showed at low concentration a differentiating effect in endothelial cells but an apoptotic effect at higher concentration (Magnelli L et al., J Nat Prod 2010, 73, 523).
Gossypol, a sesquiterpene dimer found in cotton that is formed from two cadinane units. All the cotton plant contains gossypol. That terpene occurs as a mixture of two enantiomers but each has different biological activities. For nonruminant animals (rodents, chickens, humans), (â)-gossypol is significantly more toxic than the (+) enantiomer. It has anti-cancer properties and inhibits male fertility in humans. In contrast, cotton plants containing high levels of (+)-gossypol are resistant to insect damage. These terpenes must be removed from the plant parts and oil before use as animal foods.
Bicyclic sesquiterpenes with a driman unit are widespread in plants, liveworts, fungi and certain marine organisms (sponges) (Jansen BJ et al., Nat Prod Rep 2004, 21, 449). Some drimanes have been identified in petroleum and are probably derived from a microbial source (Noble RA et al., Org Geochem 1987, 11, 151).
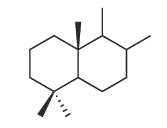
Driman squeleton
They have generally potent antibacterial and antifungal activities, and they are toxic to several invertebrates and also in fish. In addition, they deter feeding by insects on plants and by fish on sponges.
Capsidiol is a sesquiterpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. It is considered as a phytoalexin.
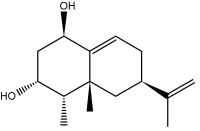
Capsidiol
Avarol, and its quinone derivative avarone, are biologically active sesquiterpenoids which exhibited antimicrobial and antifungal activities and also active against AIDS virus. They were isolated from a Mediterranean (Dysidia avara) and an Australian sponge (Dysidia spp). They have also potent antileukemic activity both in vitro and in vivo in mice.
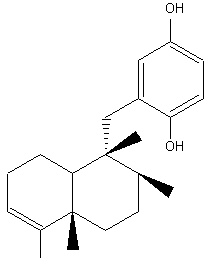
Avarol
The 1,4-benzoquinone moietylinked to the sesquiterpene is a common structural feature in a large number of terpenoid compounds which have a large spectrum of biological activities. Besides avarol, several other natural quinones and hydroquinones, such as illimaquinone, nakijiquinone and bolinaquinone, are present in sponges of the order Dictyoceratida. All have cytotoxic and antiproliferative properties which offer promising opportunities for the development of new antitumor agents. A review of the cytotoxic termene quinones from marine sponges has been released by Gordaliza M (Mar Drugs 2010, 8, 2849).
Rasmann E et al. (Nature 2005, 434, 732) discovered that insect-induced (E)-b-caryophyllene emission from maize roots attracts nematodes that prey on the attacking insect larvae. This volatile terpene is thus highly attractive to the entomopathogenic nematode which parasitizes and kills the larvae within a few days.
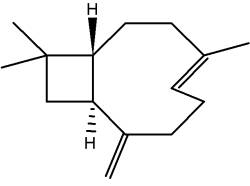
(E)-b-Caryophyllene
Caryophyllene is a constituent of many essential oils, especially clove oil (flowers of Syzygium aromaticum), the essential oil of Cannabis sativa, rosemary, and hops. It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and a-humulene. It contributes to the spiciness of black pepper.
It was shown that it is also a selective agonist of cannabinoid receptor type-2 (CB2) and thus could exert significant cannabimimetic antiinflammatory effects.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire