
PROSTANOIDS
AND
RELATED COMPOUNDS
A large number of fatty acid derivatives often present in tiny concentrations were discovered to have profound effects on cellular physiological or pathophysiological reactions. Because of their diversity, these compounds have been classified according to the type of their enzymatic formation.
Thus, the term "prostanoids" relates to the products of the cyclooxygenase pathway ( prostaglandins, prostacyclins, and thromboxanes) but to compounds produced by free radical-catalyzed mechanism (Isoprotanes, phytoprostanes, neuroprostanes).
The lipoxygenase products include leukotrienes, lipoxins, and various peroxy- or hydroxy-fatty acid derivatives. Furthermore, the term "eicosanoids" is used as a collective name for molecules derived from 20-carbon fatty acids which may belong to all the groups cited previously. Fatty acids of the n-6 family deriving from linoleic acid are the main source of eicosanoids, arachidonic acid (20:4n-6) being the major precursor. Some other 20-carbon acids (20:3n-6 and 20:5n-3) form also important metabolites which have different pharmacological properties. Eicosanoids are synthesized in vivo through several routes, some compounds being formed by more than one mechanism. In the plant kingdom, several potent derivatives from linolenic acid (octadecanoid-derived compounds) are found and have hormone-like functions (phytohormones).
FREE RADICAL-INITIATED PEROXIDATION PRODUCTS
Cyclooxygenase is the first step of a cascade giving rise to a variety of molecules containing prostanoic acid as the central structural element. For that reason, they are named prostanoids. This term includes prostaglandins, prostacyclins, thromboxanes, and related substances, but "prostaglandins" is often used loosely to include all prostanoids. Prostaglandin-like compounds (isoprostanes, phytoprostanes, isofuranes) are formed by free radical mechanisms independent of the cyclooxygenase reaction.
Prostanoids are mainly found in free forms but several combined structures are also described :
– free prostanoids
– prostanoid derivatives (amides, esters, aldehyde)
Prostaglandins were discovered in the seminal plasma around 1930 by their physiological properties and were recognized as lipids by Ulf von Euler in 1935 who was awarded the Nobel Prize in 1970 for his investigations on neurotransmitters (Nobel prize with B Katz and J Axelrod "for their discoveries concerning the humoral transmittors in the nerve terminals and the mechanism for their storage, release and inactivation". The chemical structure of prostaglandins was revealed by SK Bergstrom in 1962.
Bergstrom laid the groundwork for the current development by isolating the first prostaglandins, showing too that originated from unsaturated fatty acids. His student, BI Samuelsson, isolated and determined the structure of several of most significant prostaglandins while JR Vane discovered prostacyclin. All three were recipients of the Nobel Prize for physiology and medicine in 1982. A brief history of these discoveries may be found on the J Biol Chem internet site.
In 1969, unusually high levels of certain prostaglandins were discovered in an invertebrate animal, the octocoral Plexaura hornomella (Weinheimer AJ et al., Tetrahedron Lett 1969, 59, 5185). These high levels may have to do with chemical defense against predatory coral fish events. The physiological and ecological significance of eicosanoids in invertebrates (protozoa and metazoa) has been reviewed (Stanley DW et al., Amer Zool 1998, 38, 369). Progressively, studies have demonstrated that pathogenic fungi, protozoa, and parasitic worms have also the ability to produce eicosanoids (Noverr MC et al., Inf Immun 2002, 70, 400).
In 1970, 14 natural prostaglandins were known, actually, with the help of efficient and sensitive analytical techniques, hundreds of these compounds have been described and their number is continually growing.
To summarize the nomenclature, some indications are given below: the carbon numbering system of the mother molecule and the code of ring variants.
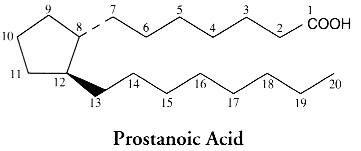
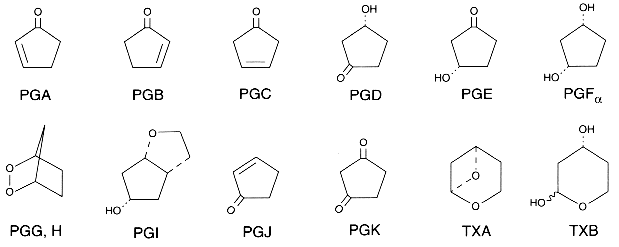
The nomenclature of PGs is based on:
– the letter component (PGA, PGB, PGC…) which identifies the functional groups of the cyclopentane ring (see the previous picture).
– the numerical subscript (PGA1, PGA2…) which recalls the number of double bonds in the carbon chains. This number depends on the the precursor fatty acid, PGE1 deriving from 20:3(n-6), PGE2 from 20:4(n-6) and PGE3 from 20:5(n-3).
To summarize the cyclooxygenase metabolism, prostaglandin G/H synthase catalyzes the sequential formation of PGG2 and PGH2 (they are both named endoperoxides) by addition of molecular oxygen at the C9, C11 and C15 positions.
|
|
|
PGH2 is then enzymatically and non-enzymatically converted in several molecules, biologically active but precursors of other active molecules. Thus, are formed PGD2 (the principal cyclooxygenase product of the mast cells and the nervous system, it inhibits platelet aggregation), PGE2 (produced in near all cell types, elevates cAMP levels, stimulates bone resorption), PGF2a, PGI2 (prostacyclin, characterizes endothelial cells, induces vasodilation and inhibits platelet aggregation), thromboxane A2, TXA2, (was first isolated from thrombocytes or platelets, it has strong platelet-aggregating activity) and even a 17 -carbon hydroxylated fatty acid 12-HHT (12-hydroxyheptadeca-5,8,10-trienoic acid), stimulates prostacyclin production and has chemotactic activity. The physiological activation of human platelets was shown to acutely mediates the esterification of free prostaglandins to membrane phospholipids (mainly phosphatidylethanolamine) (Aldrovandi M et al., J Lipid Res 2013, 54, 3085). The identification of these new metabolites opens the way for the study of how phospholipid-bound prostaglandins may play a role during platelet function.
PGE2 has been isolated from Japanese red alga Gracilaria verrucosa as a result of studies aimed at determining the cause of a human intoxication syndrome named "Ogonori poisoning" (Fusetani N et al., Bull Jpn Soc Sci Fish 1984, 50, 465). PGA2 was also isolated from Gracilaria verrucosa but PGE2 was the more potent toxin.
It is now proved that PGE2 generated in macrophages of the liver and lungs triggers the earliest phase of fever following any infection (Romanovsky AA et al., Cell Cycle 2006, 5, 2195). The following phases are known to be mediated by PGE2 originated in endotheliocytes and perivascular cells of the brain (Matsumura K et al., Front Biosci 2004, 9, 2819). PGD2, supplied by the metabolism of the n-6 polyunsaturated fatty acid arachidonic acid, regulates the recruitment of flowing neutrophils by endothelial cells stimulated with the inflammatory cytokine tumour necrosis factor-alpha (Tull SB et al., PloS Biol 2009, 7(8)).
|
|
|
|
|
|
|
|
|
Besides these important compounds and among the numerous existing metabolites, it must be cited prostaglandins of the A and B series produced from PGE series and TXB2 (stable but biologically inactive) formed from TXA2 which, as the other bioactive lipids, has a very short half-life in the tissues (some minutes).
A graphical chart of the metabolism of the eicosanoids may be found on the BioCarta web site.
Between 1975 and 1981, the formation of prostaglandin-like molecules was several times reported to be the result of peroxidation in vitro of arachidonic acid and by a free radical mechanism independent of the cyclooxygenase. Later, in 1990, prostaglandin F2-like compounds were discovered in human (Morrow JD et al., Proc Natl Acad Sci USA 1990, 87, 9383). These compounds were shown to be generated in vivo as phospholipid esters through non-enzymatic rearrangements of stereo and structural isomers of PGH2 derivatives that are produced through free radical-induced cyclooxygenation of arachidonic acid.
As these compounds are isomeric to cyclooxygenase-derived PGFa, they were termed F2-isoprostanes (F2-IsoP) (or iso-prostaglandins) and in contrast to cyclooxygenase derived prostaglandins, they are formed in situ esterified in phospholipids and are subsequently released, presumably by phospholipases (Morrow JD et al. Proc Natl Acad Sci 1990, 89, 10720).
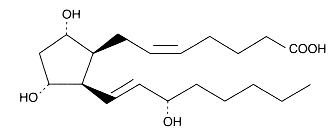
An example of the isoprostane family, 8-iso Prostaglandin F2a
or 9,11,15-trihydroxy-prosta-5,13-dien-1-oic acid.
F2-IsoP were shown to be specific and reliable markers of oxidative stress in vivo. Furthermore, they are also agonists of important biological effects, such as vasoconstriction in the kidney, in retina and brain. It was also shown that they can mediate hepatic stellate cell proliferation and collagen hyperproduction (Comporti M et al., Free Rad Biol Med 2008, 44, 247).
PGD2-like and PGE2-like compounds (iso-prostaglandins) were later shown to be produced in vivo by the same pathway (Morrow JD et al., J Biol Chem 1994, 269, 4317). Interest in these molecules stems from the fact that they may participate as patho-physiological mediators in oxidant injury and that they may also provide a valuable index of free radical-induced lipid peroxidation. Thus, 8-epi PGF2a, product of arachidonic acid, is a useful noninvasive biomarker of membrane lipid peroxidation (detected in higher amounts in plasma of smokers) and is found esterified or in free state. It has also potent vasoconstriction properties in lung and vascular tissue. Evidence was also provided that one E2-isoprostane (8-iso-prostaglandin E2) was formed in CC4 treated rat (Morrow JD et al. J Lipid Res 1998, 39, 1589).
Several regioisomers belonging to the F3-isoprotane series have been described to be generated as peroxidation products of the eicosapentaenoic acid (20:5n-3). One of the most abundant F3-isoprotanes is shown below. As these compounds are detectable in urine, they may be used as biomarkers of lipid peroxidation (Song WL et al., J Biol Chem 2009, 284, 23636).
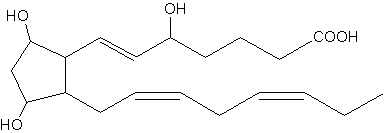
5-epi-8,12-iso-iPF3a
Similarly, it has been described that the fatty acid 22:4n-6 (adrenic acid) is susceptible to free radical attack and to yield products, F2-dihomo-IsoPs, that are similar to F2-IsoPs generated from arachidonic acid (VanRollins M et al., J Lipid Res 2008, 49, 995).
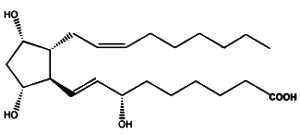
One of the F2-dihomo-isoprostanes
As adrenic acid is enriched in primate and human myelin, it was proposed that these compounds may serve as quantitative in vivo biomarkers of free radical damage to brain white matter.
Isoprostanes are determined sometimes by RIA technology but more precisely by gas chromatography-mass spectrometry.
A new classification of the possible isoprostanes was recently proposed taking into account the different precursor molecules (Lawson JA et al., J Biol Chem 1999, 274, 24441). Reviews of the general biochemistry of isoprostane pathways (Roberts LJ et al., Chem Phys Lipids 2004, 128, 173) and of the human biochemistry of isoprostane pathway (Milne GL et al., J Biol Chem 2008, 283, 15533) may be consulted. A review of the lipidomic approaches to measuring isoprostanes may be consulted (Davies SS, Eur J Lipid Sci Technol 2009, 111, 64). An historical account of their discovery and their formation have been reviewed in 2009 (Roberts LJ et al., J Lipid Res 2009, 50, S219).
Phytoprostanes : A new series of isoprostanes was recently described in plant cells, the phytoprostanes. These compounds may be considered as oxylipins, but, while they are similar to jasmonic acid, they are formed via a non- enzymatic free radical catalyzed pathway (Durand T et al., Lipids 2009, 44, 875).
Parchmann S et al. described the formation of new dinor isoprostanes E1 from linolenic acid (18:3n-3) in plants (Parchmann S et al., J Biol Chem 1998, 273, 32650). Later, a series of dinor isoprostanes F1, termed phytoprostanes F1, were shown to be formed also by non-enzymatic oxidation of linolenate (Imbusch R et al., Free Rad Biol Med 2000, 28, 720).
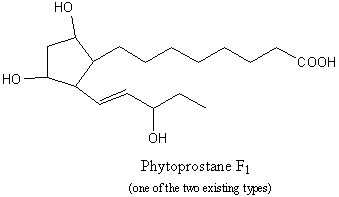
These compounds were found in free and esterified forms in roots (Valeriana), leaves (Mentha, Betula), flowers (Tilia). The total concentrations range from 0.50 to more than 10 mg/g of dry weight. During the drying of plant organs, the phytoprostane levels increased by up to 260-fold. Curiously, high levels were also found in birch pollen (Betula) (more than 32 mg/g of dry weight). It might be possible that these compounds may cause after oral absorption of vegetable food or pollen inhalation, as the related prostaglandins and the isoprostanes F2, irritations in intrapulmonary airways (Durand T et al., Biochimie 2011, 93, 52). Some experiments suggest a possible function of phytoprostanes as mediators of defense reactions in response to oxidative stress in plants. Thus, it appears that phytoprostanes may be archetypal mediators of oxidative stress: they trigger the first adaptive responses thus limiting the consequences of oxidative stress by inducing several plant-protection mechanisms.
A new phytoprostane (chromomoric acid) has been isolated from a plant used in Vietnam traditional medicine, Chromolaena odorata. It seemed to have the same property of boosting deficient detoxification capacity in plants as in mammals (Heiss EH et al., J Nat Prod 2014, 77, 503-8).
Reviews of their biosynthesis and functions were released by Thoma I et al. (Thoma I et al., Chem Phys Lipids 2004, 128, 135) and Durand T (Durand T et al., Lipids 2009, 44, 875).
A new prostane ring system has been proposed to prevent confusion in the nomenclature (Jahn U et al., Prost Leukotr Essent Fatty Acids 2010, 82, 83). New chemical strategies have led to the synthesis of enantiomerically pure B1-, F1- and E1-phytoprostane (Pinot E et al., J Org Chem 2008, 73, 3063).
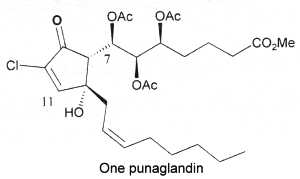
In 1985, cyclopentenone prostaglandins chlorinated at the endocyclic a-carbon position were first isolated from an octocoral Telesto riisei (Telestidae) collected in Hawaï (Baker BJ et al., J Am Chem Soc 1985, 107, 2976). They were named punaglandins and were shown to exhibit anti-inflammatory and antitumor activity likely through an inhibition of the ubiquitin-proteasome pathway (Verbitski SM et al., J Med Chem 2004, 47, 2062). Thus, this family of chlorinated lipids could offer new approaches to cancer chemotherapy.
In 2002 new arachidonic acid peroxidation products were discovered and as they are related to the isoprostanes and chemically characterized by a substituted tetrahydrofuran ring structure, they were named isofurans (Fessel JP et al., PNAS 2002, 99, 16713). These are produced in vivo by a free radical mechanism independent of the cyclooxygenase enzymes. Isofurans are present normal fluids and tissues, but levels increase dramatically in CCl4-treated rats, an animal model of oxidant injury.
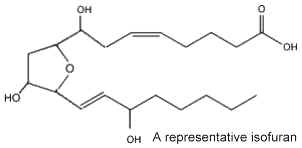
It was determined that in contrast to isoprostanes, the formation of isofurans is favored as tissue oxygen tension increases, the ratio of isofurans to isoprostanes varying according to normal steady-state tissue oxygenation. Furthermore, isofuran concentration was shown to be more elevated in nervous tissue from patients with Parkinson’s disease than in controls (Fessel JP et al., J Neurochem 2003, 85, 645).
Each prostanoid has a unique activity profile not exactly overlapping with others, indicating that each prostanoid has a specific site of action. Physiological and pharmacological studies led to the suggestion of the presence of multiple types of prostanoid receptors in different tissues and cells to a proposal to classify the prostanoid receptors in 1982 (Kennedy I et al., Prostaglandins 1982, 24, 667).
Amide derivatives
Novel amide derivatives of prostaglandins, prostaglandin ethanolamides (or prostamides) were shown to be formed by cyclooxygenase (COX-2) with anandamide as substrate, prostaglandin E2 being the major prostanoid product produced by human COX-2 (Yu M et al., J Biol Chem 1997, 272, 21181).
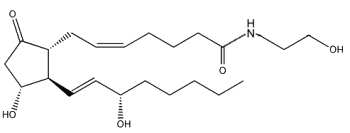
Anandamide (arachidonoyl ethanolamide) must be previously cleaved from a phosphatidylethanolamine derivative by the action of phospholipase D in response to various stimuli.
Later metabolic studies have shown that COX-2 was also able to generate ethanolamides of PGE2, PGD2 and PGF2a (Kozak KR et al., J Biol Chem 2002, 277, 44877). It was also shown that endocannabinoid-derived prostanoids of the D-, E-, I-series and thromboxane are generated by the sequential actions of COX-2 and the corresponding prostaglandin synthase at rates comparable to those observed with the presumed natural substrate, arachidonic acid (Kozak KR et al., J Biol Chem 2002, 277, 44877).
Prostamides were shown to have prominent pharmacological actions possibly by interaction with novel receptors (Matias I et al., J Pharmacol Exp Therap 2004, 309, 745). The in vivo formation of prostamides D2, F2a and E2 has been investigated using fatty acid hydrolase knockout mice (Weber A et al., J Lipid Res 2004, 45, 757). Synthetic analogues of endogenous prostamides, as Bimatoprost, are in development as hypotensive agent for the treatment of glaucoma and ocular hypertension (Cantor LB, Expert Opin Investig Drugs 2001, 10, 721)
Ester derivatives
It was demonstrated in 2000 that cyclooxygenase-2 was able to oxygenate the endocannabinoid, 2-arachidonoylglycerol, to glyceryl prostaglandins with intact macrophages or with purified enzyme, 2-acylglycerol being the preferred substrate.
The first metabolite, prostaglandin H2 glycerol ester is a substrate for cellular PGD synthase leading to prostaglandin D2 and E2 esters (PGE2-G).
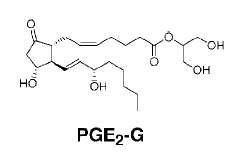
It was also shown that endocannabinoid-derived prostanoids of the D-, E-, I-series and thromboxane are generated by the sequential actions of COX-2 and the corresponding prostaglandin synthase at rates comparable to those observed with the presumed natural substrate, arachidonic acid (Kozak KR et al., J Biol Chem 2002, 277, 44877).
Pharmacological studies revealed that macrophage production of these compounds is calcium-dependent and mediated by diacylglycerol lipase and COX-2 (Kozak KR et al., J Biol Chem 2000, 275, 33744). Later, it was shown that PGE2-G was able to trigger specifically calcium mobilization, inositol-P3 synthesis, and activation of protein kinase C in macrophage cells (Nirodi C et al., Proc Natl Acad Sci USA 2004, 101, 1840).
Aldehyde derivatives
PGH2 produced by the cyclooxygenase action on arachidonic acid was shown to rearrange nonenzymatically to generate named levuglandins (LG) (secoprostanoic acid levulinaldehydes) (Salomon RG et al., J Am Chem Soc 1984, 106, 6049). These aldehydes were shown to react with lysyl residues on proteins to form stable adducts which are able to form intra- and intermolecular protein-protein cross-links (Brame CJ et al., J Biol Chem 1999, 274, 13139). It has been demonstrated that pyridoxamine, a vitamin B6 vitamer, is able to scavenge lipid-derived ketoaldehydes and then to protect cells against H2O2-mediated cytotoxicity (Davies SS et al., Biochemistry 2006, 45, 15756).
LG adducts are considered one of the first modifications of plasma lipropoteins (LDL) during their free radical oxidation (Hoppe G et al., Biochimica Biophys Acta 1997, 1344, 1). These lipid-derived proteins may serve as dosimeters of oxidative injury since it was determined that elevated plasma levels of isoLG-protein epitopes were associated with atherosclerosis independently of total cholesterol (Salomon RG, Ann N Y Acad Sci 2005, 1043, 327). It has been also determined that cellular phosphatidylethanolamine is a significant target of levuglandins and isoketals and their regio-isomers, and that formation of phosphatidylethanolamine adducts may mediate some of the biological effects of levuglandins relevant to cellular dysfunction and cell death during cardiovascular diseases (Sullivan CB et al., J Lipid Res 2010, 51, 999).
Two main levuglandins were studied, LGD2 whose structure is similar to PGE2 and LGD2 similar to PGE2.
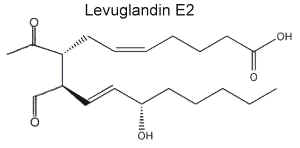
Later, it was shown that the two stereoisomers LGD2 and LGE2 may be produced by the cyclooxygenase pathway but also by the isoprostane pathway (rearrangement of isoprostane endoperoxides). Furthermore, the isoprostane pathway was shown to produce several structural isomers of levuglandins with the same chemical properties which were named isolevuglandins. The biosynthesis of isolevuglandins and the mechanism of their adduction to proteins have been extensively reviewed (Salomon RG, Chem Phys Lipids 2005, 134, 1).
The free radical-induced peroxidation of DHA may lead after molecular rearrangement via the neuroprostane pathway to the formation of highly reactive g-keto-aldehydes (Bernoud-Hubac N et al., J Biol Chem 2001, 276, 30964). These compounds were named isoketals (or neuroketals) to distinguish them from levuglandins formed by rearrangement of the cyclooxygenase endoperoxide. Eight regioisomers may be formed (each with eight racemic diastereomers). Of these eight regioisomers, four have a 1,4-pentadiene structure and four have a 1,4,7-octatriene structure on one of the side chains.
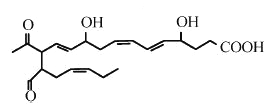
One neuroketal
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire